Sunday Poster Session
Category: IBD
P1153 - Malnutrition Is Associated With Early Colectomy Rates in Patients With Acute Severe Ulcerative Colitis Receiving Infliximab Rescue Therapy
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall

Luke Irwin, MD (he/him/his)
Scripps Mercy Hospital
San Diego, CA
Presenting Author(s)
Luke Irwin, MD1, Sang Hee Choi, MD2, Olivia Lanser, MD3, Rohit Khanna, DO3, Lori Beeken, RD4, Thien Nguyen, PharmD4, Amy Lightner, MD5, Robert Brookover, MD4, Keith Beiermeister, MD6, M. Jonathan Worsey, MD4, Lynn Weston, MD6, Melissa Ferrari, PA-C, MPAS6, Mazer Ally, MD7, Rebecca Matro, MD4, Maitreyi Kothandaraman, MD8, James Lewis, MD, MSCE9, Gauree Konijeti, MD, MPH, FACG6
1Scripps Mercy Hospital, San Diego, CA; 2Scripps Green Hospital, San Diego, CA; 3Scripps Green Hospital, La Jolla, CA; 4Scripps Health, San Diego, CA; 5Scripps Clinic, La Jolla, CA; 6Scripps Clinic Medical Group, La Jolla, CA; 7Scripps Clinic Medical Group, San Diego, CA; 8University of Calgary, San Diego, CA; 9University of Penn, Philadelphia, PA
Introduction: Malnutrition is common in patients with acute severe ulcerative colitis (ASUC) and is associated with worse clinical and perioperative outcomes. This study aims to assess the impact of nutritional status on clinical outcomes of hospitalized ASUC patients receiving infliximab (IFX) rescue therapy.
Methods: We conducted a retrospective cohort study of hospitalized patients aged ≥17 years old who received IFX rescue therapy for ASUC between 1/2017 and 12/2024 in a single health system. On admission, patients were assessed using the Malnutrition Screening Tool (MST), which assesses body mass index, unintentional weight loss, and poor p.o. intake related to acute illness. Risk for malnutrition (RM) was established by the Registered Dietitian (RD) as low (MST score = 0-1), medium (MST score = 2), or high (MST score ≥ 3), with higher scores indicating greater malnutrition risk. The primary outcome was short-term colectomy rates based on RM. Subanalyses examined IFX dosing strategy, rates of RD consultation, diet recommendations, and long-term colectomy rates.
Results: A total of 69 patients received IFX rescue therapy for ASUC, with 38% identified as high RM (n=26) and 62% (n=43) as low or medium RM. No significant differences were observed in baseline C-reactive protein, albumin, or Mayo endoscopic score. Use of standard vs. accelerated IFX dosing was similar between groups (p=0.92). Patients with high RM were more likely to receive RD consultation (p< 0.001), lactose-free diet (p=0.019) and oral nutrition supplementation (p=0.035). High RM, compared to low or medium RM, was associated with a significantly increased risk of inpatient colectomy (15% vs. 0%, p=0.017) and 3-month colectomy (23% vs. 5%, p=0.045) (Figure 1). Long-term colectomy rates at 6 months (p=0.16) and 12 months (p=0.36) were higher in the high RM group, but this was not statistically significant.
Discussion: Patients with ASUC identified as high malnutrition risk had significantly increased inpatient and 3-month colectomy rates after receiving IFX rescue therapy. Optimizing nutritional status early in ASUC hospitalization may improve outcomes and reduce colectomy rates.
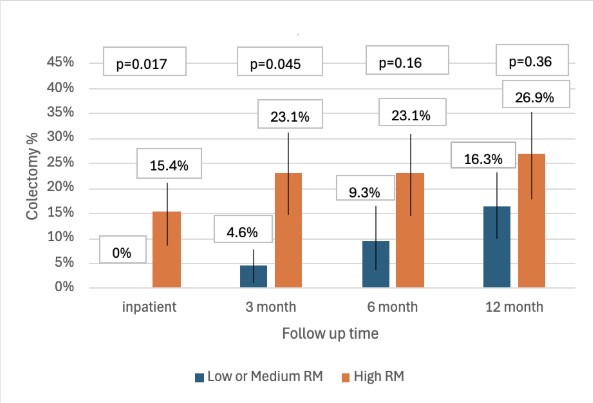
Figure: Colectomy rates according to risk of malnutrition
Disclosures:
Luke Irwin indicated no relevant financial relationships.
Sang Hee Choi indicated no relevant financial relationships.
Olivia Lanser indicated no relevant financial relationships.
Rohit Khanna indicated no relevant financial relationships.
Lori Beeken indicated no relevant financial relationships.
Thien Nguyen indicated no relevant financial relationships.
Amy Lightner indicated no relevant financial relationships.
Robert Brookover indicated no relevant financial relationships.
Keith Beiermeister indicated no relevant financial relationships.
M. Jonathan Worsey indicated no relevant financial relationships.
Lynn Weston indicated no relevant financial relationships.
Melissa Ferrari: Abbvie – Speakers Bureau. Eli Lilly – Advisory Committee/Board Member. Johnson and Johnson – Speakers Bureau. Pfizer – Advisory Committee/Board Member.
Mazer Ally: Abbvie – Speakers Bureau. Lilly – Advisory Committee/Board Member.
Rebecca Matro indicated no relevant financial relationships.
Maitreyi Kothandaraman indicated no relevant financial relationships.
James Lewis: 3M – Expert witness. AbbVie – Grant/Research Support. Amgen – Advisor or Review Panel Member. Dark Canyon Laboratories – Owner/Ownership Interest. Eli Lilly – Consultant, Grant/Research Support. Johnson & Johnson – Advisory Committee/Board Member, Grant/Research Support. Odyssey Therapeutics – Advisor or Review Panel Member. Pfizer – Advisor or Review Panel Member. Sanofi – Advisor or Review Panel Member. Spyre Therapeutics – Advisor or Review Panel Member.
Gauree Konijeti: Abbvie – Advisory Committee/Board Member, Consultant. Johnson and Johnson – Consultant. Lilly – Consultant, Speakers Bureau. Pfizer – Advisory Committee/Board Member. Takeda – Speakers Bureau. WellTheory – Consultant, Stock Options.
Luke Irwin, MD1, Sang Hee Choi, MD2, Olivia Lanser, MD3, Rohit Khanna, DO3, Lori Beeken, RD4, Thien Nguyen, PharmD4, Amy Lightner, MD5, Robert Brookover, MD4, Keith Beiermeister, MD6, M. Jonathan Worsey, MD4, Lynn Weston, MD6, Melissa Ferrari, PA-C, MPAS6, Mazer Ally, MD7, Rebecca Matro, MD4, Maitreyi Kothandaraman, MD8, James Lewis, MD, MSCE9, Gauree Konijeti, MD, MPH, FACG6. P1153 - Malnutrition Is Associated With Early Colectomy Rates in Patients With Acute Severe Ulcerative Colitis Receiving Infliximab Rescue Therapy, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Scripps Mercy Hospital, San Diego, CA; 2Scripps Green Hospital, San Diego, CA; 3Scripps Green Hospital, La Jolla, CA; 4Scripps Health, San Diego, CA; 5Scripps Clinic, La Jolla, CA; 6Scripps Clinic Medical Group, La Jolla, CA; 7Scripps Clinic Medical Group, San Diego, CA; 8University of Calgary, San Diego, CA; 9University of Penn, Philadelphia, PA
Introduction: Malnutrition is common in patients with acute severe ulcerative colitis (ASUC) and is associated with worse clinical and perioperative outcomes. This study aims to assess the impact of nutritional status on clinical outcomes of hospitalized ASUC patients receiving infliximab (IFX) rescue therapy.
Methods: We conducted a retrospective cohort study of hospitalized patients aged ≥17 years old who received IFX rescue therapy for ASUC between 1/2017 and 12/2024 in a single health system. On admission, patients were assessed using the Malnutrition Screening Tool (MST), which assesses body mass index, unintentional weight loss, and poor p.o. intake related to acute illness. Risk for malnutrition (RM) was established by the Registered Dietitian (RD) as low (MST score = 0-1), medium (MST score = 2), or high (MST score ≥ 3), with higher scores indicating greater malnutrition risk. The primary outcome was short-term colectomy rates based on RM. Subanalyses examined IFX dosing strategy, rates of RD consultation, diet recommendations, and long-term colectomy rates.
Results: A total of 69 patients received IFX rescue therapy for ASUC, with 38% identified as high RM (n=26) and 62% (n=43) as low or medium RM. No significant differences were observed in baseline C-reactive protein, albumin, or Mayo endoscopic score. Use of standard vs. accelerated IFX dosing was similar between groups (p=0.92). Patients with high RM were more likely to receive RD consultation (p< 0.001), lactose-free diet (p=0.019) and oral nutrition supplementation (p=0.035). High RM, compared to low or medium RM, was associated with a significantly increased risk of inpatient colectomy (15% vs. 0%, p=0.017) and 3-month colectomy (23% vs. 5%, p=0.045) (Figure 1). Long-term colectomy rates at 6 months (p=0.16) and 12 months (p=0.36) were higher in the high RM group, but this was not statistically significant.
Discussion: Patients with ASUC identified as high malnutrition risk had significantly increased inpatient and 3-month colectomy rates after receiving IFX rescue therapy. Optimizing nutritional status early in ASUC hospitalization may improve outcomes and reduce colectomy rates.

Figure: Colectomy rates according to risk of malnutrition
Disclosures:
Luke Irwin indicated no relevant financial relationships.
Sang Hee Choi indicated no relevant financial relationships.
Olivia Lanser indicated no relevant financial relationships.
Rohit Khanna indicated no relevant financial relationships.
Lori Beeken indicated no relevant financial relationships.
Thien Nguyen indicated no relevant financial relationships.
Amy Lightner indicated no relevant financial relationships.
Robert Brookover indicated no relevant financial relationships.
Keith Beiermeister indicated no relevant financial relationships.
M. Jonathan Worsey indicated no relevant financial relationships.
Lynn Weston indicated no relevant financial relationships.
Melissa Ferrari: Abbvie – Speakers Bureau. Eli Lilly – Advisory Committee/Board Member. Johnson and Johnson – Speakers Bureau. Pfizer – Advisory Committee/Board Member.
Mazer Ally: Abbvie – Speakers Bureau. Lilly – Advisory Committee/Board Member.
Rebecca Matro indicated no relevant financial relationships.
Maitreyi Kothandaraman indicated no relevant financial relationships.
James Lewis: 3M – Expert witness. AbbVie – Grant/Research Support. Amgen – Advisor or Review Panel Member. Dark Canyon Laboratories – Owner/Ownership Interest. Eli Lilly – Consultant, Grant/Research Support. Johnson & Johnson – Advisory Committee/Board Member, Grant/Research Support. Odyssey Therapeutics – Advisor or Review Panel Member. Pfizer – Advisor or Review Panel Member. Sanofi – Advisor or Review Panel Member. Spyre Therapeutics – Advisor or Review Panel Member.
Gauree Konijeti: Abbvie – Advisory Committee/Board Member, Consultant. Johnson and Johnson – Consultant. Lilly – Consultant, Speakers Bureau. Pfizer – Advisory Committee/Board Member. Takeda – Speakers Bureau. WellTheory – Consultant, Stock Options.
Luke Irwin, MD1, Sang Hee Choi, MD2, Olivia Lanser, MD3, Rohit Khanna, DO3, Lori Beeken, RD4, Thien Nguyen, PharmD4, Amy Lightner, MD5, Robert Brookover, MD4, Keith Beiermeister, MD6, M. Jonathan Worsey, MD4, Lynn Weston, MD6, Melissa Ferrari, PA-C, MPAS6, Mazer Ally, MD7, Rebecca Matro, MD4, Maitreyi Kothandaraman, MD8, James Lewis, MD, MSCE9, Gauree Konijeti, MD, MPH, FACG6. P1153 - Malnutrition Is Associated With Early Colectomy Rates in Patients With Acute Severe Ulcerative Colitis Receiving Infliximab Rescue Therapy, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
