Sunday Poster Session
Category: IBD
P1148 - Baseline Characteristics Associated With Probability of Remission and Greater Absolute Benefit of Treatment With Afimkibart (RO7790121/RG6631)
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall

Jessica R. Allegretti, MD, MPH, FACG (she/her/hers)
Division of Gastroenterology, Hepatology, and Endoscopy, Brigham and Women’s Hospital, Harvard Medical School
Boston, MA
Presenting Author(s)
Jessica R.. Allegretti, MD, MPH1, Laurent Peyrin-Biroulet, MD, PhD2, Silvio Danese, MD, PhD3, Parambir S. Dulai, MD4, Anindita Banerjee, PhD5, Deepa E. Chandra, MPharm5, Elena Peeva, MD5, Srividya Neelakantan, PhD5, Michael S. Vincent, MD, PhD5, Kenneth Hung, MD, PhD5, Lyann Ursos, PhD6, Courtney Schiffman, PhD6, Daniela Bojic, MD, PhD7, Karen Lasch, MD6, Bruce E. Sands, MD, MS, FACG8
1Division of Gastroenterology, Hepatology, and Endoscopy, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA; 2Department of Gastroenterology, CHRU Nancy, INSERM NGERE, Université de Lorraine, France, Vandœuvre-lès-Nancy, Lorraine, France; 3Gastroenterology and Endoscopy, IRCCS Ospedale San Raffaele and University Vita-Salute San Raffaele, Milan, Lombardia, Italy; 4Division of Gastroenterology and Hepatology, Feinberg School of Medicine, Northwestern University, Chicago, IL; 5Pfizer Inc., Cambridge, MA; 6Genentech, Inc., a member of the Roche Group, South San Francisco, CA; 7F. Hoffmann-La Roche Ltd, Basel, Basel-Stadt, Switzerland; 8Dr. Henry D. Janowitz Division of Gastroenterology, Icahn School of Medicine at Mount Sinai, New York, NY, USA, New York, NY
Introduction: In the phase IIb TUSCANY-2 study (NCT04090411), afimkibart (RO7790121/RG6631), an anti-TL1A antibody, had a clinically meaningful effect in patients (pts) with moderate to severe ulcerative colitis. We report post hoc analyses of the relationship between baseline clinical characteristics and Week 14 (W14) efficacy in pts enrolled in TUSCANY-2.
Methods: Pts were randomized to receive subcutaneous afimkibart 50mg, 150mg, 450mg or placebo (PBO) monthly during induction. In this analysis, afimkibart doses were pooled; the endpoint was clinical remission by modified Mayo score (mMS; defined as stool frequency subscore = 0/1; rectal bleeding subscore = 0; and endoscopic subscore = 0/1) at W14. Investigation of patient-specific treatment effects was guided by the risk modeling approach from the Predictive Approaches to Treatment effect Heterogeneity statement.1 We developed a regularized logistic regression model to predict the probability of W14 remission using baseline mMS, C-reactive protein (CRP), serum albumin, Robart’s Histopathology Index (RHI), number of failed advanced therapy classes, corticosteroid use, disease extent, disease duration, age and sex. The model was used to separate patients by baseline clinical characteristics to interrogate variation in treatment effects.
Results: Overall, 32.5% of pts achieved clinical remission by mMS at W14 in the afimkibart arm (65/200; 90% CI 27.3–38.1) vs 13.3% (6/45; 90% CI 7.1–23.8) with PBO (treatment Δ 19.2%; 90% CI 7.6–27.9). Based on calibrated predictions from the multivariate model, all pts (PBO and afimkibart arms) had a 2–76% probability of W14 remission at baseline. On the clinically meaningful absolute scale, the treatment Δ in the top 50% of pts with a higher baseline probability of remission (27–76%) was 28.5% (90% CI 8–42; 45.2% afimkibart vs 16.7% PBO), versus a treatment Δ of 7.6% (90% CI –7–18; 18.8% afimkibart vs 11.1% PBO) in pts with a lower baseline probability of remission (2–27%; Figure). Lower baseline CRP, lower RHI and higher serum albumin contributed to a greater probability of remission and absolute benefit with afimkibart.
Discussion: This analysis demonstrates how precision medicine approaches that simultaneously account for diverse baseline characteristics may improve interpretation of positive inflammatory bowel disease (IBD) clinical trial results. Integrating genetic and novel biomarkers with clinical features could help to better predict outcomes for pts with IBD.
1. Kent D, et al. Ann Intern Med 2020;172:35–45
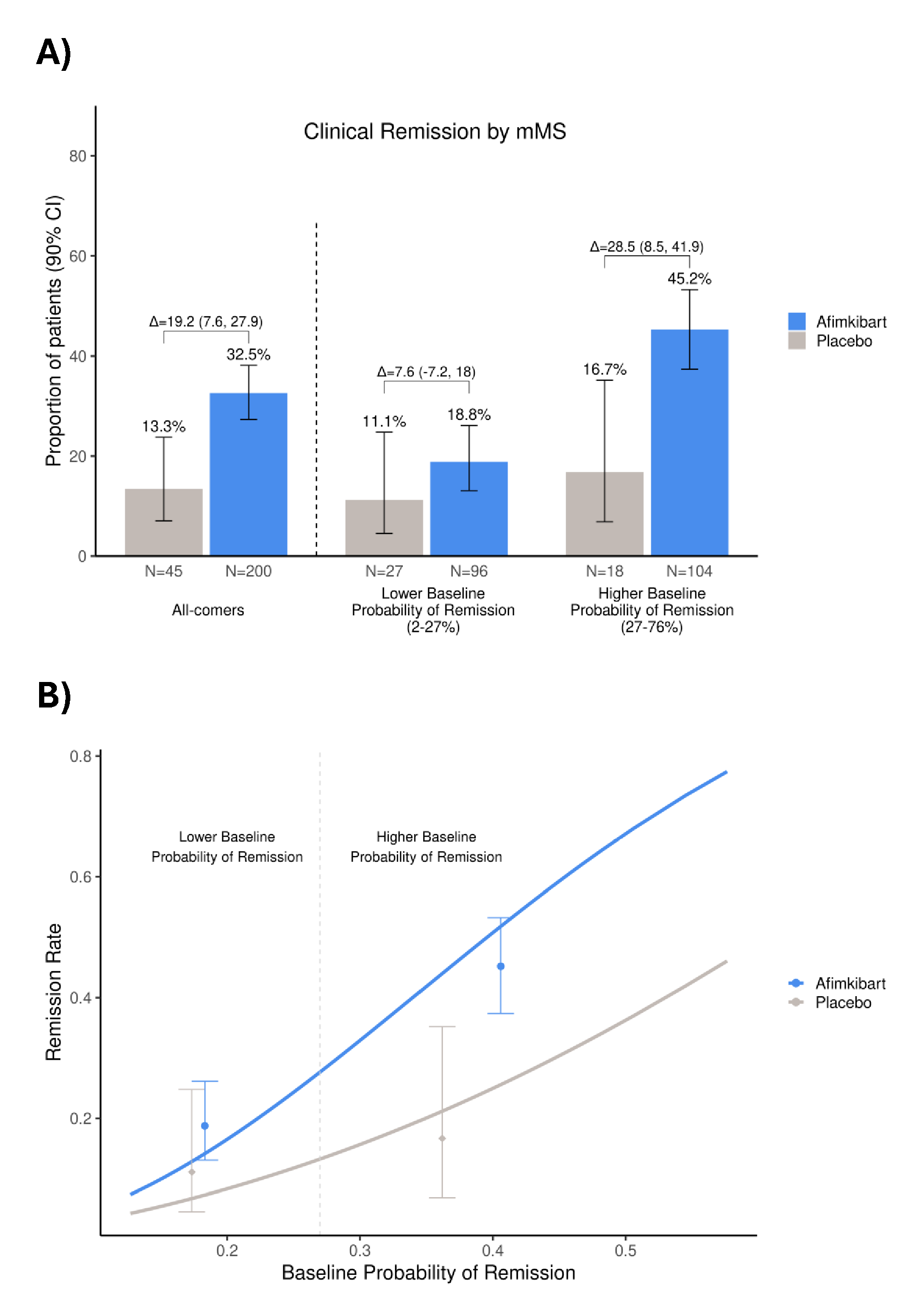
Figure: Figure 1. The proportion of patients who achieved clinical remission by mMS, categorized by baseline probability of remission (A) the impact of baseline probability of clinical remission by mMS versus observed remission rate in patients (B)
Error bars represent A) 90% CI of the proportion of patients who achieved clinical remission
B) 90% CI of the remission rate
CI, confidence interval; mMS, modified Mayo Score
Disclosures:
Jessica Allegretti: Abbvie – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Speaker, Speakers Bureau. Adiso – Consultant. Bristol Myer Squibb – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Speaker. Celltrion – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. Ferring – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. Finch – Consultant. Genentech – Advisory Committee/Board Member, Consultant. GlaxoSmithKline – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. Iterative Scopes – Consultant. Janssen – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Speakers Bureau. Johnson & Johnson – Consultant, Grant/Research Support, Speaker. Merck – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Grant/Research Support. Pfizer – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Grant/Research Support. Roivant – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. Roivant Adiso – Consultant. Seres Therapeutics – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. Shattuck Labs – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. TRXBio – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. Vedanta – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant.
Laurent Peyrin-Biroulet: AbbVie – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, meeting attendance/travel support, Speakers Bureau. Abivax – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Adacyte – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. Alfasigma – Speakers Bureau. Alimentiv – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Amgen – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, meeting attendance/travel support, Speakers Bureau. Applied Molecular Transport – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Arena – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, Speakers Bureau. Banook – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Biogen – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, Speakers Bureau. Bristol Myers Squibb – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Celltrion – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Grant/Research Support, Honoraria, meeting attendance/travel support, Speakers Bureau. Connect Biopharm – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, meeting attendance/travel support. Cytoki Pharma – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Eli Lilly – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, meeting attendance/travel support, Speakers Bureau. Enthera – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. F. Hoffmann-La Roche Ltd – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Ferring – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, meeting attendance/travel support, Speakers Bureau. Fresenius Kabi – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Grant/Research Support, Honoraria. Galapagos – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, meeting attendance/travel support, Speakers Bureau. Genentech – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, meeting attendance/travel support, Speakers Bureau. Gilead – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, meeting attendance/travel support, Speakers Bureau. Gossamer Bio – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, meeting attendance/travel support. GSK – Advisory Committee/Board Member, Consultant. IAC Image Analysis – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Index Pharmaceuticals – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Inotrem – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Janssen – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Speakers Bureau. Johnson & Johnson – Advisory Committee/Board Member, Consultant, Honoraria, meeting attendance/travel support. Medac – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Grant/Research Support, Honoraria, meeting attendance/travel support, Speakers Bureau. Mopac – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Morphic – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, meeting attendance/travel support. MSD – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Grant/Research Support, Honoraria, meeting attendance/travel support, Speakers Bureau. Nordic Pharma – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, Speakers Bureau. Novartis – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Oncodesign Precision Medicine – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. ONO Pharma – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. OSE Immunotherapeutics – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Pandion Therapeutics – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Par' Immune – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Pfizer – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, meeting attendance/travel support, Speakers Bureau. Prometheus – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Protagonist – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Samsung – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Sandoz – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, meeting attendance/travel support, Speakers Bureau. Sanofi – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Satisfay – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Takeda – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Grant/Research Support, Honoraria, meeting attendance/travel support, Speakers Bureau. Telavant – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Theravance – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Thermo Fischer – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, meeting attendance/travel support. Tigenix – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Tillots – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, meeting attendance/travel support, Speakers Bureau. Vectivbio – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Ventyx – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Viatris – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, Speakers Bureau. Ysopia – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria.
Silvio Danese: AbbVie – Consultant, Lecture fees. Alimentiv – Consultant. Allergan – Consultant. Amgen – Consultant, Lecture fees. AstraZeneca – Consultant. Athos Therapeutics – Consultant. Biogen – Consultant. Boehringer Ingelheim – Consultant. Celgene – Consultant. Celltrion – Consultant. Eli Lilly – Consultant. Enthera – Consultant. F. Hoffmann-La Roche Ltd – Consultant. Ferring Pharmaceuticals Inc. – Consultant, Lecture fees. Gilead – Consultant, Lecture fees. Hospira – Consultant. Inotrem – Consultant. Johnson & Johnson – Consultant, Lecture fees. MSD – Consultant. Mundipharma – Consultant. Mylan – Consultant, Lecture fees. Pfizer – Consultant, Lecture fees. Sandoz – Consultant. Sublimity Therapeutics – Consultant. Takeda – Consultant, Lecture fees. TiGenix – Consultant. UCB Inc. – Consultant. Vifor (International) Ltd. – Consultant.
Parambir S. Dulai: AbbVie – Consultant. Abivax – Consultant. Adiso – Consultant. Alimentiv – Consultant. Bristol Myers Squibb – Consultant. Celltrion – Consultant. Digbi Health – Stock Options. Genentech – Consultant. Geneoscopy – Consultant. GSK – Consultant. Janssen – Consultant. Lilly – Consultant. Pfizer – Consultant, Grant/Research Support. Precidiag – Royalties. Takeda – Consultant, Grant/Research Support.
Anindita Banerjee: Pfizer Inc. – Employee, Stock Options.
Deepa E. Chandra: Pfizer Inc. – Employee, Stock Options.
Elena Peeva: Pfizer Inc. – Employee, Royalties, Stock Options.
Srividya Neelakantan: Pfizer Inc. – Employee, Stock Options.
Michael S. Vincent: Pfizer Inc. – Employee, Stock Options.
Kenneth Hung indicated no relevant financial relationships.
Lyann Ursos: Genentech, a member of the Roche Group – Employee.
Courtney Schiffman: Genentech, a member of the Roche Group – Employee, Stock Options.
Daniela Bojic: F. Hoffmann-La Roche Ltd – Employee.
Karen Lasch: Genentech, a member of the Roche Group – Employee, Stock Options.
Bruce Sands: AbbVie – Consultant. Abivax – Consultant, speaking fees. Adiso Therapeutics – Consultant. Agomab Therapeutics – Consultant. Alimentiv – Consultant. Amgen – Consultant. AnaptysBio – Consultant. Arena Pharmaceuticals – Consultant. Artugen Therapeutics – Consultant. Astra Zeneca – Consultant. Biolojic Design – Consultant. Biora Therapeutics – Consultant. Boehringer Ingelheim – Consultant. Boston Pharmaceuticals – Consultant. Bristol Myers Squibb – Consultant, Grant/Research Support, speaking fees. Calibr – Consultant. Celgene – Consultant. Celltrion – Consultant. ClostraBio – Consultant. Eli Lilly & Company – Consultant, speaking fees. Enthera – Consultant. Enveda Biosciences – Consultant. Equillium – Consultant. Evommune – Consultant. Ferring – Consultant. Fresenius Kabi – Consultant. Fzatat – Consultant. Galapagos – Consultant. Genentech (Roche) – Consultant. Gilead Sciences – Consultant. GlaxoSmithKline – Consultant. Gossamer Bio – Consultant. Imhotex – Consultant. Index Pharmaceuticals – Consultant. Innovation Pharmaceuticals – Consultant. Inotrem – Consultant. Janssen R&D – Consultant, Grant/Research Support, speaking fees. Kaleido – Consultant. Kallyope – Consultant. Merck & Co., Inc., Rahway, NJ, USA – Consultant. Microba – Consultant. Mobius Care – Consultant. Morphic Therapeutics – Consultant. MRM Health – Consultant. Nexus Therapeutics – Consultant. Nimbus Discovery – Consultant. Odyssey Therapeutics – Consultant. Pfizer – Consultant, Grant/Research Support, speaking fees. Progenity – Consultant. Prometheus Biosciences – Consultant. Prometheus Laboratories – Consultant. Protagonist Therapeutics – Consultant. Q32 Bio – Consultant. Rasayana Therapeutics – Consultant. Recludix Therapeutics – Consultant. Reistone Biopharma – Consultant. Sanofi – Consultant. Spyre Therapeutics – Consultant. Sun Pharma – Consultant. Surrozen – Consultant. Takeda – Consultant, Grant/Research Support, speaking fees. Target RWE – Consultant. Teva – Consultant. Theravance Biopharma – Consultant, Grant/Research Support. TLL Pharmaceutical – Consultant. Tr1X – Consultant. Union Therapeutics – Consultant. Ventyx Biosciences – Consultant, Stock Options.
Jessica R.. Allegretti, MD, MPH1, Laurent Peyrin-Biroulet, MD, PhD2, Silvio Danese, MD, PhD3, Parambir S. Dulai, MD4, Anindita Banerjee, PhD5, Deepa E. Chandra, MPharm5, Elena Peeva, MD5, Srividya Neelakantan, PhD5, Michael S. Vincent, MD, PhD5, Kenneth Hung, MD, PhD5, Lyann Ursos, PhD6, Courtney Schiffman, PhD6, Daniela Bojic, MD, PhD7, Karen Lasch, MD6, Bruce E. Sands, MD, MS, FACG8. P1148 - Baseline Characteristics Associated With Probability of Remission and Greater Absolute Benefit of Treatment With Afimkibart (RO7790121/RG6631), ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Division of Gastroenterology, Hepatology, and Endoscopy, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA; 2Department of Gastroenterology, CHRU Nancy, INSERM NGERE, Université de Lorraine, France, Vandœuvre-lès-Nancy, Lorraine, France; 3Gastroenterology and Endoscopy, IRCCS Ospedale San Raffaele and University Vita-Salute San Raffaele, Milan, Lombardia, Italy; 4Division of Gastroenterology and Hepatology, Feinberg School of Medicine, Northwestern University, Chicago, IL; 5Pfizer Inc., Cambridge, MA; 6Genentech, Inc., a member of the Roche Group, South San Francisco, CA; 7F. Hoffmann-La Roche Ltd, Basel, Basel-Stadt, Switzerland; 8Dr. Henry D. Janowitz Division of Gastroenterology, Icahn School of Medicine at Mount Sinai, New York, NY, USA, New York, NY
Introduction: In the phase IIb TUSCANY-2 study (NCT04090411), afimkibart (RO7790121/RG6631), an anti-TL1A antibody, had a clinically meaningful effect in patients (pts) with moderate to severe ulcerative colitis. We report post hoc analyses of the relationship between baseline clinical characteristics and Week 14 (W14) efficacy in pts enrolled in TUSCANY-2.
Methods: Pts were randomized to receive subcutaneous afimkibart 50mg, 150mg, 450mg or placebo (PBO) monthly during induction. In this analysis, afimkibart doses were pooled; the endpoint was clinical remission by modified Mayo score (mMS; defined as stool frequency subscore = 0/1; rectal bleeding subscore = 0; and endoscopic subscore = 0/1) at W14. Investigation of patient-specific treatment effects was guided by the risk modeling approach from the Predictive Approaches to Treatment effect Heterogeneity statement.1 We developed a regularized logistic regression model to predict the probability of W14 remission using baseline mMS, C-reactive protein (CRP), serum albumin, Robart’s Histopathology Index (RHI), number of failed advanced therapy classes, corticosteroid use, disease extent, disease duration, age and sex. The model was used to separate patients by baseline clinical characteristics to interrogate variation in treatment effects.
Results: Overall, 32.5% of pts achieved clinical remission by mMS at W14 in the afimkibart arm (65/200; 90% CI 27.3–38.1) vs 13.3% (6/45; 90% CI 7.1–23.8) with PBO (treatment Δ 19.2%; 90% CI 7.6–27.9). Based on calibrated predictions from the multivariate model, all pts (PBO and afimkibart arms) had a 2–76% probability of W14 remission at baseline. On the clinically meaningful absolute scale, the treatment Δ in the top 50% of pts with a higher baseline probability of remission (27–76%) was 28.5% (90% CI 8–42; 45.2% afimkibart vs 16.7% PBO), versus a treatment Δ of 7.6% (90% CI –7–18; 18.8% afimkibart vs 11.1% PBO) in pts with a lower baseline probability of remission (2–27%; Figure). Lower baseline CRP, lower RHI and higher serum albumin contributed to a greater probability of remission and absolute benefit with afimkibart.
Discussion: This analysis demonstrates how precision medicine approaches that simultaneously account for diverse baseline characteristics may improve interpretation of positive inflammatory bowel disease (IBD) clinical trial results. Integrating genetic and novel biomarkers with clinical features could help to better predict outcomes for pts with IBD.
1. Kent D, et al. Ann Intern Med 2020;172:35–45

Figure: Figure 1. The proportion of patients who achieved clinical remission by mMS, categorized by baseline probability of remission (A) the impact of baseline probability of clinical remission by mMS versus observed remission rate in patients (B)
Error bars represent A) 90% CI of the proportion of patients who achieved clinical remission
B) 90% CI of the remission rate
CI, confidence interval; mMS, modified Mayo Score
Disclosures:
Jessica Allegretti: Abbvie – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Speaker, Speakers Bureau. Adiso – Consultant. Bristol Myer Squibb – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Speaker. Celltrion – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. Ferring – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. Finch – Consultant. Genentech – Advisory Committee/Board Member, Consultant. GlaxoSmithKline – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. Iterative Scopes – Consultant. Janssen – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Speakers Bureau. Johnson & Johnson – Consultant, Grant/Research Support, Speaker. Merck – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Grant/Research Support. Pfizer – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Grant/Research Support. Roivant – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. Roivant Adiso – Consultant. Seres Therapeutics – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. Shattuck Labs – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. TRXBio – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. Vedanta – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant.
Laurent Peyrin-Biroulet: AbbVie – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, meeting attendance/travel support, Speakers Bureau. Abivax – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Adacyte – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. Alfasigma – Speakers Bureau. Alimentiv – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Amgen – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, meeting attendance/travel support, Speakers Bureau. Applied Molecular Transport – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Arena – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, Speakers Bureau. Banook – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Biogen – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, Speakers Bureau. Bristol Myers Squibb – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Celltrion – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Grant/Research Support, Honoraria, meeting attendance/travel support, Speakers Bureau. Connect Biopharm – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, meeting attendance/travel support. Cytoki Pharma – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Eli Lilly – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, meeting attendance/travel support, Speakers Bureau. Enthera – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. F. Hoffmann-La Roche Ltd – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Ferring – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, meeting attendance/travel support, Speakers Bureau. Fresenius Kabi – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Grant/Research Support, Honoraria. Galapagos – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, meeting attendance/travel support, Speakers Bureau. Genentech – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, meeting attendance/travel support, Speakers Bureau. Gilead – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, meeting attendance/travel support, Speakers Bureau. Gossamer Bio – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, meeting attendance/travel support. GSK – Advisory Committee/Board Member, Consultant. IAC Image Analysis – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Index Pharmaceuticals – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Inotrem – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Janssen – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Speakers Bureau. Johnson & Johnson – Advisory Committee/Board Member, Consultant, Honoraria, meeting attendance/travel support. Medac – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Grant/Research Support, Honoraria, meeting attendance/travel support, Speakers Bureau. Mopac – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Morphic – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, meeting attendance/travel support. MSD – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Grant/Research Support, Honoraria, meeting attendance/travel support, Speakers Bureau. Nordic Pharma – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, Speakers Bureau. Novartis – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Oncodesign Precision Medicine – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. ONO Pharma – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. OSE Immunotherapeutics – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Pandion Therapeutics – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Par' Immune – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Pfizer – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, meeting attendance/travel support, Speakers Bureau. Prometheus – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Protagonist – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Samsung – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Sandoz – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, meeting attendance/travel support, Speakers Bureau. Sanofi – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Satisfay – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Takeda – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Grant/Research Support, Honoraria, meeting attendance/travel support, Speakers Bureau. Telavant – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Theravance – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Thermo Fischer – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, meeting attendance/travel support. Tigenix – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Tillots – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, meeting attendance/travel support, Speakers Bureau. Vectivbio – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Ventyx – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Viatris – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, Speakers Bureau. Ysopia – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria.
Silvio Danese: AbbVie – Consultant, Lecture fees. Alimentiv – Consultant. Allergan – Consultant. Amgen – Consultant, Lecture fees. AstraZeneca – Consultant. Athos Therapeutics – Consultant. Biogen – Consultant. Boehringer Ingelheim – Consultant. Celgene – Consultant. Celltrion – Consultant. Eli Lilly – Consultant. Enthera – Consultant. F. Hoffmann-La Roche Ltd – Consultant. Ferring Pharmaceuticals Inc. – Consultant, Lecture fees. Gilead – Consultant, Lecture fees. Hospira – Consultant. Inotrem – Consultant. Johnson & Johnson – Consultant, Lecture fees. MSD – Consultant. Mundipharma – Consultant. Mylan – Consultant, Lecture fees. Pfizer – Consultant, Lecture fees. Sandoz – Consultant. Sublimity Therapeutics – Consultant. Takeda – Consultant, Lecture fees. TiGenix – Consultant. UCB Inc. – Consultant. Vifor (International) Ltd. – Consultant.
Parambir S. Dulai: AbbVie – Consultant. Abivax – Consultant. Adiso – Consultant. Alimentiv – Consultant. Bristol Myers Squibb – Consultant. Celltrion – Consultant. Digbi Health – Stock Options. Genentech – Consultant. Geneoscopy – Consultant. GSK – Consultant. Janssen – Consultant. Lilly – Consultant. Pfizer – Consultant, Grant/Research Support. Precidiag – Royalties. Takeda – Consultant, Grant/Research Support.
Anindita Banerjee: Pfizer Inc. – Employee, Stock Options.
Deepa E. Chandra: Pfizer Inc. – Employee, Stock Options.
Elena Peeva: Pfizer Inc. – Employee, Royalties, Stock Options.
Srividya Neelakantan: Pfizer Inc. – Employee, Stock Options.
Michael S. Vincent: Pfizer Inc. – Employee, Stock Options.
Kenneth Hung indicated no relevant financial relationships.
Lyann Ursos: Genentech, a member of the Roche Group – Employee.
Courtney Schiffman: Genentech, a member of the Roche Group – Employee, Stock Options.
Daniela Bojic: F. Hoffmann-La Roche Ltd – Employee.
Karen Lasch: Genentech, a member of the Roche Group – Employee, Stock Options.
Bruce Sands: AbbVie – Consultant. Abivax – Consultant, speaking fees. Adiso Therapeutics – Consultant. Agomab Therapeutics – Consultant. Alimentiv – Consultant. Amgen – Consultant. AnaptysBio – Consultant. Arena Pharmaceuticals – Consultant. Artugen Therapeutics – Consultant. Astra Zeneca – Consultant. Biolojic Design – Consultant. Biora Therapeutics – Consultant. Boehringer Ingelheim – Consultant. Boston Pharmaceuticals – Consultant. Bristol Myers Squibb – Consultant, Grant/Research Support, speaking fees. Calibr – Consultant. Celgene – Consultant. Celltrion – Consultant. ClostraBio – Consultant. Eli Lilly & Company – Consultant, speaking fees. Enthera – Consultant. Enveda Biosciences – Consultant. Equillium – Consultant. Evommune – Consultant. Ferring – Consultant. Fresenius Kabi – Consultant. Fzatat – Consultant. Galapagos – Consultant. Genentech (Roche) – Consultant. Gilead Sciences – Consultant. GlaxoSmithKline – Consultant. Gossamer Bio – Consultant. Imhotex – Consultant. Index Pharmaceuticals – Consultant. Innovation Pharmaceuticals – Consultant. Inotrem – Consultant. Janssen R&D – Consultant, Grant/Research Support, speaking fees. Kaleido – Consultant. Kallyope – Consultant. Merck & Co., Inc., Rahway, NJ, USA – Consultant. Microba – Consultant. Mobius Care – Consultant. Morphic Therapeutics – Consultant. MRM Health – Consultant. Nexus Therapeutics – Consultant. Nimbus Discovery – Consultant. Odyssey Therapeutics – Consultant. Pfizer – Consultant, Grant/Research Support, speaking fees. Progenity – Consultant. Prometheus Biosciences – Consultant. Prometheus Laboratories – Consultant. Protagonist Therapeutics – Consultant. Q32 Bio – Consultant. Rasayana Therapeutics – Consultant. Recludix Therapeutics – Consultant. Reistone Biopharma – Consultant. Sanofi – Consultant. Spyre Therapeutics – Consultant. Sun Pharma – Consultant. Surrozen – Consultant. Takeda – Consultant, Grant/Research Support, speaking fees. Target RWE – Consultant. Teva – Consultant. Theravance Biopharma – Consultant, Grant/Research Support. TLL Pharmaceutical – Consultant. Tr1X – Consultant. Union Therapeutics – Consultant. Ventyx Biosciences – Consultant, Stock Options.
Jessica R.. Allegretti, MD, MPH1, Laurent Peyrin-Biroulet, MD, PhD2, Silvio Danese, MD, PhD3, Parambir S. Dulai, MD4, Anindita Banerjee, PhD5, Deepa E. Chandra, MPharm5, Elena Peeva, MD5, Srividya Neelakantan, PhD5, Michael S. Vincent, MD, PhD5, Kenneth Hung, MD, PhD5, Lyann Ursos, PhD6, Courtney Schiffman, PhD6, Daniela Bojic, MD, PhD7, Karen Lasch, MD6, Bruce E. Sands, MD, MS, FACG8. P1148 - Baseline Characteristics Associated With Probability of Remission and Greater Absolute Benefit of Treatment With Afimkibart (RO7790121/RG6631), ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
