Sunday Poster Session
Category: IBD
P1139 - One Subcutaneous Injection of Mirikizumab Is Bioequivalent to 2 Subcutaneous Injections: Results from a Pharmacokinetic Comparability Study in Healthy Participants
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall

Yuki Otani (he/him/his)
Eli Lilly and Company
Indianapolis, IN
Presenting Author(s)
Yuki Otani, 1, Christopher Payne, 1, Edward V.. Loftus, MD2, Geert R. D’Haens, MD, PhD3, Shomron Ben Horin, 4, Abhishek Upadhya, 1, Kristin Todd, 1, Paola Pellanda, 1, Xin Zhang, 1
1Eli Lilly and Company, Indianapolis, IN; 2Mayo Clinic College of Medicine and Science and Mayo Clinic, Rochester, MN; 3Department of Gastroenterology, Amsterdam University Medical Center, Amsterdam, Noord-Holland, Netherlands; 4Sheba Medical Center, Tel-Hashomer, Affiliated with the Tel Aviv University, Tel Aviv, Tel Aviv, Israel
Introduction: Mirikizumab (miri), a humanized IgG4 monoclonal antibody that selectively binds to interleukin-23 subunit p19, is approved for moderately to severely active ulcerative colitis and Crohn’s disease. In ulcerative colitis, maintenance treatment with 200 mg miri given as two 1-mL subcutaneous (SC) injections every 4 weeks was efficacious and safe in the phase 3 maintenance LUCENT-2 and long-term extension LUCENT-3 trials. Given the pain/discomfort associated with SC injections, a single 2-mL injection delivering the same dose has been developed to enhance patient adherence.
Methods: This phase 1 study evaluated the pharmacokinetic (PK) bioequivalence and safety of miri solution administered as one 2-ml injection versus the commercially available two 1-ml injections. Healthy participants were stratified into weight categories (< 75 kg, 75–85 kg, and >85 kg) and randomized (1:1) to receive one dose of 200 mg of SC citrate-free miri as either one (200 mg/2 mL) or two injections (each of 100 mg/1 mL) delivered by an autoinjector. Participants were sub-randomized (1:1:1) by injection site (arm, thigh, and abdomen). Blood samples were collected at pre-defined intervals up to 10 weeks post-dosing. The primary PK endpoints were the maximum observed concentration (Cmax), area under the concentration–time curve (AUC) from time zero to infinity (AUC0–∞), and AUC from time zero to the last timepoint with measurable concentration (AUC0–t). The secondary endpoint was safety [treatment-emergent adverse events (TEAEs) and serious adverse events (SAEs)]. Evaluating the impact of injection site location on PK was an exploratory endpoint.
Results: Of 244 participants receiving one 2-mL injection (mean age: 42.4 years; 55.3% female) and 240 receiving two 1-mL injections (mean age: 42.7 years; 53.3% male), baseline characteristics were comparable. The three primary PK parameters all fell within the bioequivalence range (geometric least-squares mean [GM] ratio: 0.800–1.250; see table). Observed GM were consistent across injection site locations. TEAEs were reported in 48 participants with one injection (19.7%) and 55 with two injections (22.9%). Most TEAEs were mild (62/74 events with one injection [83.8%] vs 85/100 with two injections [85.0%]). No SAEs were reported in either group.
Discussion: Miri 200 mg administered as one 2-ml SC injection was bioequivalent to two 1-ml injections, and most TEAEs were mild. In clinical practice, reducing the number of injections may improve treatment adherence.
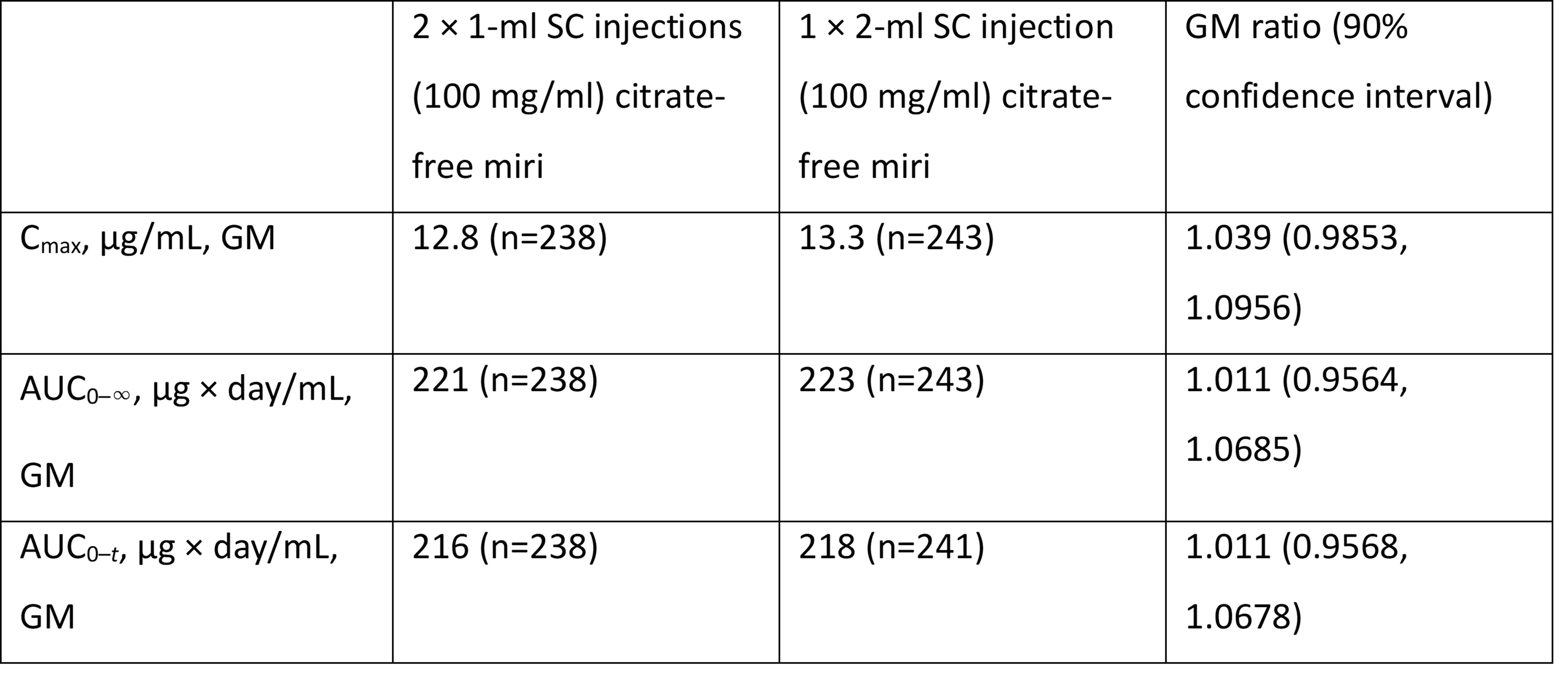
Figure: Summary of Pharmacokinetic Parameters
Disclosures:
Yuki Otani: Eli Lilly and Company – Employee, Stock Options.
Christopher Payne: Eli Lilly and Company – Employee, Stock Options.
Edward Loftus: AbbVie – Consultant, Grant/Research Support. Abivax – Consultant. Astellas – Consultant. Avalo – Consultant. Biocon – Consultant. Celltrion – Consultant. Eli Lilly – Advisory Committee/Board Member, Consultant. Exact Sciences – Stock Options. Fresenius Kabi – Consultant. Genentech – Advisory Committee/Board Member. Gilead – Consultant, Grant/Research Support. Iota Biosciences – Consultant. Iterative Health – Consultant. Johnson & Johnson – Consultant, Grant/Research Support. Merck – Consultant, Grant/Research Support. Moderna – Stock Options. Morphic – Consultant. Ono Pharma – Consultant. Spyre – Advisory Committee/Board Member. Takeda – Consultant, Grant/Research Support. TR1X Bio – Consultant.
Geert D’Haens: Abbvie – Advisor or Review Panel Member, Speakers Bureau. Abivax – Advisor or Review Panel Member. Agomab – Advisor or Review Panel Member. Alimentiv – Advisor or Review Panel Member. AMT – Consultant. Anaptys Bio – Advisor or Review Panel Member. AstraZeneca – Consultant. Boehringer Ingelheim – Advisor or Review Panel Member. Bristol Myers Squibb – Advisor or Review Panel Member, Speakers Bureau. Celltrion – Advisor or Review Panel Member. Eli Lilly – Advisor or Review Panel Member. Exeliom – Consultant. Galapagos – Advisor or Review Panel Member. Glaxo Smith Kline – Advisor or Review Panel Member. Gossamerbio – Consultant. Immunic – Consultant. Index Pharmaceuticals – Advisor or Review Panel Member. Johnson & Johnson – Advisor or Review Panel Member. Kaleido – Consultant. Merck – Advisor or Review Panel Member. Origo – Consultant. Pfizer – Consultant, Speakers Bureau. Polpharm – Advisor or Review Panel Member. Procise Diagnostics – Consultant. Progenity – Consultant. Prometheus Biosciences – Advisor or Review Panel Member. Protagonist Therapeutics – Consultant. Sanofi – Advisor or Review Panel Member. Seres Health – Advisory Committee/Board Member. Takeda – Advisor or Review Panel Member. Tillotts – Advisor or Review Panel Member.
Shomron Ben Horin: Abbvie – Advisory Committee/Board Member, Consultant, Grant/Research Support. Alma Therapeutics – Stock Options. Celltrion – Advisory Committee/Board Member, Consultant, Grant/Research Support. Eli Lilly and Company – Advisory Committee/Board Member, Consultant. Evinature – Stock Options. Ferring – Advisory Committee/Board Member, Consultant. Galmed – Advisory Committee/Board Member, Consultant, Grant/Research Support, Stock Options. Gilead – Advisory Committee/Board Member, Consultant. GSK – Advisory Committee/Board Member, Consultant. Janssen – Advisory Committee/Board Member, Consultant, Grant/Research Support. Medial Earlysign – Advisory Committee/Board Member, Consultant. Medtronic – Grant/Research Support. NeoPharm – Advisory Committee/Board Member, Consultant. Novartis – Advisory Committee/Board Member, Consultant. Pfizer – Advisory Committee/Board Member, Consultant, Grant/Research Support. Predicta Med – Advisory Committee/Board Member, Consultant. PredictaMed – Stock Options. Roche – Advisory Committee/Board Member, Consultant. Takeda – Advisory Committee/Board Member, Consultant, Grant/Research Support.
Abhishek Upadhya: Eli Lilly and Company – Employee, Stock Options.
Kristin Todd: Eli Lilly and Company – Employee, Stock Options.
Paola Pellanda: Eli Lilly and Company – Employee, Stock Options.
Xin Zhang: Eli Lilly and Company – Employee, Stock Options.
Yuki Otani, 1, Christopher Payne, 1, Edward V.. Loftus, MD2, Geert R. D’Haens, MD, PhD3, Shomron Ben Horin, 4, Abhishek Upadhya, 1, Kristin Todd, 1, Paola Pellanda, 1, Xin Zhang, 1. P1139 - One Subcutaneous Injection of Mirikizumab Is Bioequivalent to 2 Subcutaneous Injections: Results from a Pharmacokinetic Comparability Study in Healthy Participants, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Eli Lilly and Company, Indianapolis, IN; 2Mayo Clinic College of Medicine and Science and Mayo Clinic, Rochester, MN; 3Department of Gastroenterology, Amsterdam University Medical Center, Amsterdam, Noord-Holland, Netherlands; 4Sheba Medical Center, Tel-Hashomer, Affiliated with the Tel Aviv University, Tel Aviv, Tel Aviv, Israel
Introduction: Mirikizumab (miri), a humanized IgG4 monoclonal antibody that selectively binds to interleukin-23 subunit p19, is approved for moderately to severely active ulcerative colitis and Crohn’s disease. In ulcerative colitis, maintenance treatment with 200 mg miri given as two 1-mL subcutaneous (SC) injections every 4 weeks was efficacious and safe in the phase 3 maintenance LUCENT-2 and long-term extension LUCENT-3 trials. Given the pain/discomfort associated with SC injections, a single 2-mL injection delivering the same dose has been developed to enhance patient adherence.
Methods: This phase 1 study evaluated the pharmacokinetic (PK) bioequivalence and safety of miri solution administered as one 2-ml injection versus the commercially available two 1-ml injections. Healthy participants were stratified into weight categories (< 75 kg, 75–85 kg, and >85 kg) and randomized (1:1) to receive one dose of 200 mg of SC citrate-free miri as either one (200 mg/2 mL) or two injections (each of 100 mg/1 mL) delivered by an autoinjector. Participants were sub-randomized (1:1:1) by injection site (arm, thigh, and abdomen). Blood samples were collected at pre-defined intervals up to 10 weeks post-dosing. The primary PK endpoints were the maximum observed concentration (Cmax), area under the concentration–time curve (AUC) from time zero to infinity (AUC0–∞), and AUC from time zero to the last timepoint with measurable concentration (AUC0–t). The secondary endpoint was safety [treatment-emergent adverse events (TEAEs) and serious adverse events (SAEs)]. Evaluating the impact of injection site location on PK was an exploratory endpoint.
Results: Of 244 participants receiving one 2-mL injection (mean age: 42.4 years; 55.3% female) and 240 receiving two 1-mL injections (mean age: 42.7 years; 53.3% male), baseline characteristics were comparable. The three primary PK parameters all fell within the bioequivalence range (geometric least-squares mean [GM] ratio: 0.800–1.250; see table). Observed GM were consistent across injection site locations. TEAEs were reported in 48 participants with one injection (19.7%) and 55 with two injections (22.9%). Most TEAEs were mild (62/74 events with one injection [83.8%] vs 85/100 with two injections [85.0%]). No SAEs were reported in either group.
Discussion: Miri 200 mg administered as one 2-ml SC injection was bioequivalent to two 1-ml injections, and most TEAEs were mild. In clinical practice, reducing the number of injections may improve treatment adherence.

Figure: Summary of Pharmacokinetic Parameters
Disclosures:
Yuki Otani: Eli Lilly and Company – Employee, Stock Options.
Christopher Payne: Eli Lilly and Company – Employee, Stock Options.
Edward Loftus: AbbVie – Consultant, Grant/Research Support. Abivax – Consultant. Astellas – Consultant. Avalo – Consultant. Biocon – Consultant. Celltrion – Consultant. Eli Lilly – Advisory Committee/Board Member, Consultant. Exact Sciences – Stock Options. Fresenius Kabi – Consultant. Genentech – Advisory Committee/Board Member. Gilead – Consultant, Grant/Research Support. Iota Biosciences – Consultant. Iterative Health – Consultant. Johnson & Johnson – Consultant, Grant/Research Support. Merck – Consultant, Grant/Research Support. Moderna – Stock Options. Morphic – Consultant. Ono Pharma – Consultant. Spyre – Advisory Committee/Board Member. Takeda – Consultant, Grant/Research Support. TR1X Bio – Consultant.
Geert D’Haens: Abbvie – Advisor or Review Panel Member, Speakers Bureau. Abivax – Advisor or Review Panel Member. Agomab – Advisor or Review Panel Member. Alimentiv – Advisor or Review Panel Member. AMT – Consultant. Anaptys Bio – Advisor or Review Panel Member. AstraZeneca – Consultant. Boehringer Ingelheim – Advisor or Review Panel Member. Bristol Myers Squibb – Advisor or Review Panel Member, Speakers Bureau. Celltrion – Advisor or Review Panel Member. Eli Lilly – Advisor or Review Panel Member. Exeliom – Consultant. Galapagos – Advisor or Review Panel Member. Glaxo Smith Kline – Advisor or Review Panel Member. Gossamerbio – Consultant. Immunic – Consultant. Index Pharmaceuticals – Advisor or Review Panel Member. Johnson & Johnson – Advisor or Review Panel Member. Kaleido – Consultant. Merck – Advisor or Review Panel Member. Origo – Consultant. Pfizer – Consultant, Speakers Bureau. Polpharm – Advisor or Review Panel Member. Procise Diagnostics – Consultant. Progenity – Consultant. Prometheus Biosciences – Advisor or Review Panel Member. Protagonist Therapeutics – Consultant. Sanofi – Advisor or Review Panel Member. Seres Health – Advisory Committee/Board Member. Takeda – Advisor or Review Panel Member. Tillotts – Advisor or Review Panel Member.
Shomron Ben Horin: Abbvie – Advisory Committee/Board Member, Consultant, Grant/Research Support. Alma Therapeutics – Stock Options. Celltrion – Advisory Committee/Board Member, Consultant, Grant/Research Support. Eli Lilly and Company – Advisory Committee/Board Member, Consultant. Evinature – Stock Options. Ferring – Advisory Committee/Board Member, Consultant. Galmed – Advisory Committee/Board Member, Consultant, Grant/Research Support, Stock Options. Gilead – Advisory Committee/Board Member, Consultant. GSK – Advisory Committee/Board Member, Consultant. Janssen – Advisory Committee/Board Member, Consultant, Grant/Research Support. Medial Earlysign – Advisory Committee/Board Member, Consultant. Medtronic – Grant/Research Support. NeoPharm – Advisory Committee/Board Member, Consultant. Novartis – Advisory Committee/Board Member, Consultant. Pfizer – Advisory Committee/Board Member, Consultant, Grant/Research Support. Predicta Med – Advisory Committee/Board Member, Consultant. PredictaMed – Stock Options. Roche – Advisory Committee/Board Member, Consultant. Takeda – Advisory Committee/Board Member, Consultant, Grant/Research Support.
Abhishek Upadhya: Eli Lilly and Company – Employee, Stock Options.
Kristin Todd: Eli Lilly and Company – Employee, Stock Options.
Paola Pellanda: Eli Lilly and Company – Employee, Stock Options.
Xin Zhang: Eli Lilly and Company – Employee, Stock Options.
Yuki Otani, 1, Christopher Payne, 1, Edward V.. Loftus, MD2, Geert R. D’Haens, MD, PhD3, Shomron Ben Horin, 4, Abhishek Upadhya, 1, Kristin Todd, 1, Paola Pellanda, 1, Xin Zhang, 1. P1139 - One Subcutaneous Injection of Mirikizumab Is Bioequivalent to 2 Subcutaneous Injections: Results from a Pharmacokinetic Comparability Study in Healthy Participants, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
