Sunday Poster Session
Category: IBD
P1132 - Oral Immune-Related Adverse Events in Patients with and Without Inflammatory Bowel Disease Receiving Immune Checkpoint Inhibitor Therapy: A Retrospective Cohort Study
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall

Tanvi Gupta, MD
University of Texas Health Science Center
Houston, TX
Presenting Author(s)
Sharada Wali, MBBS, MPH1, Tanvi Gupta, MD2, Saltenat Moghaddam Adames, MD, MPH3, Anirudha Chatterjee, MD4, Ahmed Rehman, BS3, Mariam Rizvi, MD5, Nina Quirk, MD, MS6, Maria Julia M. N.. Santos, MD7, Carolina Cruz, MD1, Krishnavathana Varatharajalu, MD7, Anusha Shirwaikar Thomas, MD1, Yinghong Wang, MD, PhD, MS1
1University of Texas MD Anderson Cancer Center, Houston, TX; 2University of Texas Health Science Center, Houston, TX; 3Baylor College of Medicine, Houston, TX; 4University of Texas Medical Branch, Galveston, TX; 5University of Texas at Houston, Houston, TX; 6University of Texas Health Sciences Center in Houston, Houston, TX; 7MD Anderson Cancer Center, Houston, TX
Introduction: Immune checkpoint inhibitors (ICIs) can cause oral immune-related adverse events (irAEs), leading to malnutrition, dehydration, pain, and treatment delays. Due to overlapping features between IBD-related oral symptoms and oral irAEs, it is important to assess whether mucosal immune dysregulation in IBD increases the risk or severity of these toxicities. Clarifying this link may aid in early detection, risk stratification, and tailored management for IBD patients on ICI therapy.
Methods: This retrospective cohort study included adult patients who received ICIs for cancer therapy at a tertiary cancer center from 2012 to 2025. Patients who developed oral irAEs within one year of ICI initiation were grouped by IBD status, using 1:2 matching by age, sex, and cancer type. Clinical data was extracted from electronic records to compare irAE features, severity (CTCAE v5.0), and management.
Results: Among 450 ICI-treated patients, 152 had pre-existing IBD (evenly split between UC and CD), and 298 did not (Table 1). Median time to oral lesion onset was longer in IBD vs non-IBD patients (16 vs 10.1 weeks). Oral mucosal toxicities were significantly less common in the IBD group (12.2% vs 43.0%, p< 0.001; Table 2). IBD patients reported fewer symptoms: xerostomia (2% vs 22.1%, p< 0.001), dysgeusia (0% vs 14.4%, p< 0.001), mucositis (6% vs 12.1%, p=0.046), dysphagia (0.7% vs 7%, p=0.002), and oral candidiasis (0% vs 12.4%, p< 0.001). CTCAE severity grades for mucositis, oral pain, and dysphagia were similar across groups. Supportive care was the mainstay in both groups, though less frequent in IBD patients (7.3% vs 27.2%, p< 0.001). Topical antifungals were only used in non-IBD patients (12.1% vs 0%, p< 0.001). Among IBD patients, 43.4% received IBD treatment within 3 months of oral irAE onset. Hospitalization rates for oral lesions were low (2.9% in UC, 11.5% in CD). ICI therapy was held in 10.5% of IBD and 10.1% of non-IBD cases. Oral lesion recurrence was lower in IBD (6.1%) vs non-IBD (8.2%). All-cause mortality was lower in the IBD group (37.8% vs 49.2%, p=0.026) with similar follow-up durations.
Discussion: Oral irAEs were significantly less common in IBD patients on ICI therapy than in non-IBD patients. This may suggest a protective effect of ongoing IBD treatment against mucosal toxicities, as many IBD patients were receiving colitis therapy at ICI initiation. These findings support the tolerability of ICIs in IBD patients, with oral toxicities often mild and manageable with supportive care.
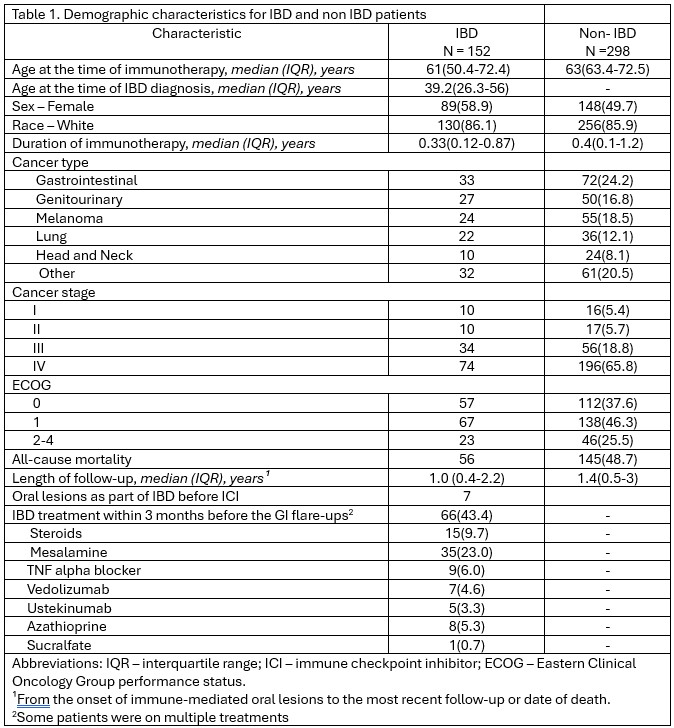
Figure: Demographic characteristics for IBD and non-IBD patients.
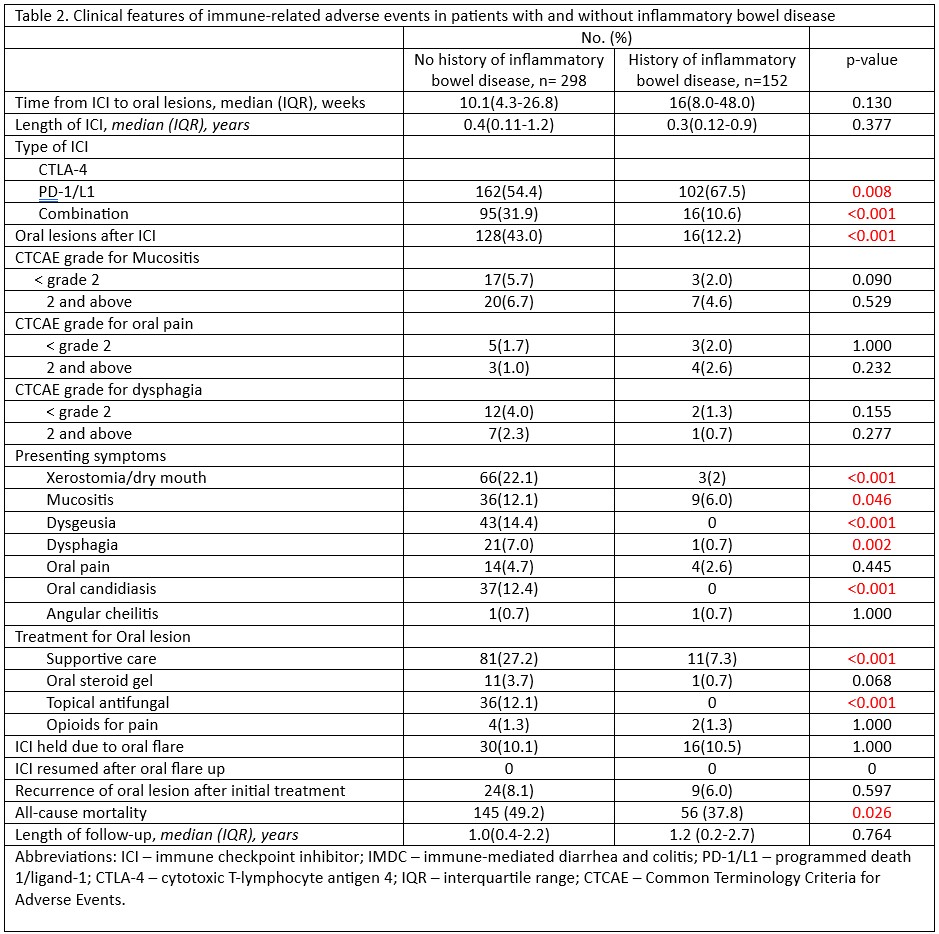
Figure: Clinical features of immune-related adverse events in patients with and without inflammatory bowel disease.
Disclosures:
Sharada Wali indicated no relevant financial relationships.
Tanvi Gupta indicated no relevant financial relationships.
Saltenat Moghaddam Adames indicated no relevant financial relationships.
Anirudha Chatterjee indicated no relevant financial relationships.
Ahmed Rehman indicated no relevant financial relationships.
Mariam Rizvi indicated no relevant financial relationships.
Nina Quirk indicated no relevant financial relationships.
Maria Julia Santos indicated no relevant financial relationships.
Carolina Cruz indicated no relevant financial relationships.
Krishnavathana Varatharajalu indicated no relevant financial relationships.
Anusha Shirwaikar Thomas indicated no relevant financial relationships.
Yinghong Wang indicated no relevant financial relationships.
Sharada Wali, MBBS, MPH1, Tanvi Gupta, MD2, Saltenat Moghaddam Adames, MD, MPH3, Anirudha Chatterjee, MD4, Ahmed Rehman, BS3, Mariam Rizvi, MD5, Nina Quirk, MD, MS6, Maria Julia M. N.. Santos, MD7, Carolina Cruz, MD1, Krishnavathana Varatharajalu, MD7, Anusha Shirwaikar Thomas, MD1, Yinghong Wang, MD, PhD, MS1. P1132 - Oral Immune-Related Adverse Events in Patients with and Without Inflammatory Bowel Disease Receiving Immune Checkpoint Inhibitor Therapy: A Retrospective Cohort Study, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1University of Texas MD Anderson Cancer Center, Houston, TX; 2University of Texas Health Science Center, Houston, TX; 3Baylor College of Medicine, Houston, TX; 4University of Texas Medical Branch, Galveston, TX; 5University of Texas at Houston, Houston, TX; 6University of Texas Health Sciences Center in Houston, Houston, TX; 7MD Anderson Cancer Center, Houston, TX
Introduction: Immune checkpoint inhibitors (ICIs) can cause oral immune-related adverse events (irAEs), leading to malnutrition, dehydration, pain, and treatment delays. Due to overlapping features between IBD-related oral symptoms and oral irAEs, it is important to assess whether mucosal immune dysregulation in IBD increases the risk or severity of these toxicities. Clarifying this link may aid in early detection, risk stratification, and tailored management for IBD patients on ICI therapy.
Methods: This retrospective cohort study included adult patients who received ICIs for cancer therapy at a tertiary cancer center from 2012 to 2025. Patients who developed oral irAEs within one year of ICI initiation were grouped by IBD status, using 1:2 matching by age, sex, and cancer type. Clinical data was extracted from electronic records to compare irAE features, severity (CTCAE v5.0), and management.
Results: Among 450 ICI-treated patients, 152 had pre-existing IBD (evenly split between UC and CD), and 298 did not (Table 1). Median time to oral lesion onset was longer in IBD vs non-IBD patients (16 vs 10.1 weeks). Oral mucosal toxicities were significantly less common in the IBD group (12.2% vs 43.0%, p< 0.001; Table 2). IBD patients reported fewer symptoms: xerostomia (2% vs 22.1%, p< 0.001), dysgeusia (0% vs 14.4%, p< 0.001), mucositis (6% vs 12.1%, p=0.046), dysphagia (0.7% vs 7%, p=0.002), and oral candidiasis (0% vs 12.4%, p< 0.001). CTCAE severity grades for mucositis, oral pain, and dysphagia were similar across groups. Supportive care was the mainstay in both groups, though less frequent in IBD patients (7.3% vs 27.2%, p< 0.001). Topical antifungals were only used in non-IBD patients (12.1% vs 0%, p< 0.001). Among IBD patients, 43.4% received IBD treatment within 3 months of oral irAE onset. Hospitalization rates for oral lesions were low (2.9% in UC, 11.5% in CD). ICI therapy was held in 10.5% of IBD and 10.1% of non-IBD cases. Oral lesion recurrence was lower in IBD (6.1%) vs non-IBD (8.2%). All-cause mortality was lower in the IBD group (37.8% vs 49.2%, p=0.026) with similar follow-up durations.
Discussion: Oral irAEs were significantly less common in IBD patients on ICI therapy than in non-IBD patients. This may suggest a protective effect of ongoing IBD treatment against mucosal toxicities, as many IBD patients were receiving colitis therapy at ICI initiation. These findings support the tolerability of ICIs in IBD patients, with oral toxicities often mild and manageable with supportive care.

Figure: Demographic characteristics for IBD and non-IBD patients.

Figure: Clinical features of immune-related adverse events in patients with and without inflammatory bowel disease.
Disclosures:
Sharada Wali indicated no relevant financial relationships.
Tanvi Gupta indicated no relevant financial relationships.
Saltenat Moghaddam Adames indicated no relevant financial relationships.
Anirudha Chatterjee indicated no relevant financial relationships.
Ahmed Rehman indicated no relevant financial relationships.
Mariam Rizvi indicated no relevant financial relationships.
Nina Quirk indicated no relevant financial relationships.
Maria Julia Santos indicated no relevant financial relationships.
Carolina Cruz indicated no relevant financial relationships.
Krishnavathana Varatharajalu indicated no relevant financial relationships.
Anusha Shirwaikar Thomas indicated no relevant financial relationships.
Yinghong Wang indicated no relevant financial relationships.
Sharada Wali, MBBS, MPH1, Tanvi Gupta, MD2, Saltenat Moghaddam Adames, MD, MPH3, Anirudha Chatterjee, MD4, Ahmed Rehman, BS3, Mariam Rizvi, MD5, Nina Quirk, MD, MS6, Maria Julia M. N.. Santos, MD7, Carolina Cruz, MD1, Krishnavathana Varatharajalu, MD7, Anusha Shirwaikar Thomas, MD1, Yinghong Wang, MD, PhD, MS1. P1132 - Oral Immune-Related Adverse Events in Patients with and Without Inflammatory Bowel Disease Receiving Immune Checkpoint Inhibitor Therapy: A Retrospective Cohort Study, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
