Sunday Poster Session
Category: IBD
P1131 - Prior Biologic or Janus Kinase Inhibitor Therapy Does Not Impact Colectomy Rates Among Patients Needing Infliximab Rescue Therapy for Acute Severe Ulcerative Colitis
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall
- SC
Sang Hee K. Choi, MD
Scripps Green Hospital
San Diego, CA
Presenting Author(s)
Sang Hee Choi, MD1, Rohit Khanna, DO2, Olivia Lanser, MD2, Luke Irwin, MD3, Thien Nguyen, PharmD4, Mazer Ally, MD5, Rebecca Matro, MD4, Amy Lightner, MD6, Robert Brookover, MD4, Keith Beiermeister, MD7, M. Jonathan Worsey, MD4, Lynn Weston, MD7, Melissa Ferrari, PA-C, MPAS7, Gauree Konijeti, MD, MPH, FACG7
1Scripps Green Hospital, San Diego, CA; 2Scripps Green Hospital, La Jolla, CA; 3Scripps Mercy Hospital, San Diego, CA; 4Scripps Health, San Diego, CA; 5Scripps Clinic Medical Group, San Diego, CA; 6Scripps Clinic, La Jolla, CA; 7Scripps Clinic Medical Group, La Jolla, CA
Introduction: Infliximab (IFX) is recommended for steroid-refractory patients hospitalized with acute severe ulcerative colitis (ASUC). However, limited data exist on its efficacy in patients with prior exposure to advanced therapies (AT), including biologics or janus kinase (JAK) inhibitors. This study examines the efficacy of IFX rescue therapy in ASUC patients with and without prior AT exposure.
Methods: We conducted a health system-wide retrospective cohort study of hospitalized patients aged ≥17 years who received inpatient IFX rescue therapy for ASUC between January 2017 and December 2024. The primary endpoint was colectomy rates among AT-naïve vs. AT-exposed patients during index hospitalization and within 3 months following inpatient IFX treatment. Secondary endpoints included the impact of inpatient IFX dosing strategy (standard vs. accelerated), post-discharge IFX persistence at 1 year, use of other AT during follow-up, and colectomy rate at 1 year.
Results: A total of 75 patients received IFX rescue therapy (AT-naïve = 52, AT-exposed = 23). Among AT-exposed, 65% had received 1 prior therapy, 30% had received 2, and 4% had received ≥ 3; 16 patients (28%) were actively on AT at the time of index hospitalization (Table 1). UC duration, CRP, and albumin levels on admission did not differ between groups. AT-naïve patients were more likely to have a Mayo endoscopic subscore of 3 (87% vs. 65% AT-exposed, p=0.03). No significant differences were observed for inpatient (p >0.99) or 3-month (p=0.16) colectomy rates between AT-naïve and AT-exposed patients (Figure 1). Infliximab dosing strategy, whether standard or accelerated (increased frequency, dosing, or both), did not differ between groups and was not independently associated with the primary outcome. AT-exposed patients were more likely to continue IFX at 1 year (52% vs 11%, p< 0.01). Colectomy rates at 1 year were similar (21.7% for AT-exposed vs. 21.2% for AT-naïve, p >0.99).
Discussion: Prior exposure to biologic or JAK inhibitors does not appear to impact short-term colectomy rates following IFX rescue therapy for ASUC, suggesting that IFX remains an effective salvage option regardless of prior treatment history. Future studies are needed to evaluate long-term clinical outcomes.
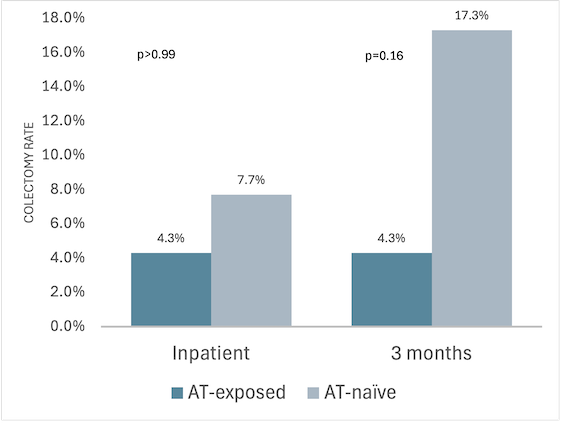
Figure: Table 1. Baseline demographic and clinical characteristics of study cohort.
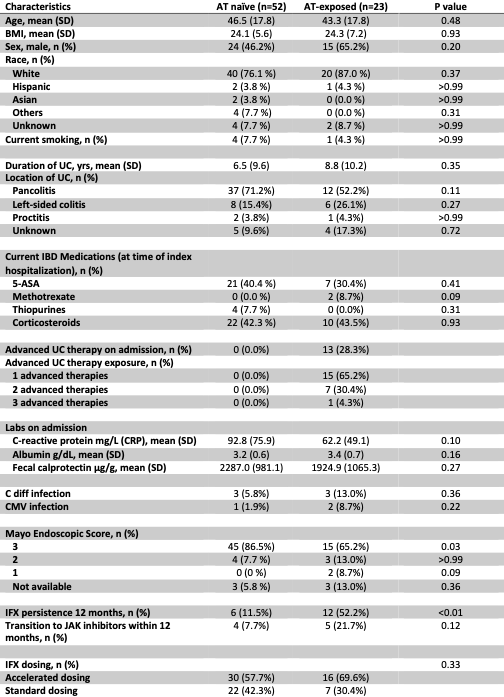
Figure: Figure 1. Colectomy rates during index hospitalization and up to 3 months in advance therapy naïve vs. exposed patients.
Disclosures:
Sang Hee Choi indicated no relevant financial relationships.
Rohit Khanna indicated no relevant financial relationships.
Olivia Lanser indicated no relevant financial relationships.
Luke Irwin indicated no relevant financial relationships.
Thien Nguyen indicated no relevant financial relationships.
Mazer Ally: Abbvie – Speakers Bureau. Lilly – Advisory Committee/Board Member.
Rebecca Matro indicated no relevant financial relationships.
Amy Lightner indicated no relevant financial relationships.
Robert Brookover indicated no relevant financial relationships.
Keith Beiermeister indicated no relevant financial relationships.
M. Jonathan Worsey indicated no relevant financial relationships.
Lynn Weston indicated no relevant financial relationships.
Melissa Ferrari: Abbvie – Speakers Bureau. Eli Lilly – Advisory Committee/Board Member. Johnson and Johnson – Speakers Bureau. Pfizer – Advisory Committee/Board Member.
Gauree Konijeti: Abbvie – Advisory Committee/Board Member, Consultant. Johnson and Johnson – Consultant. Lilly – Consultant, Speakers Bureau. Pfizer – Advisory Committee/Board Member. Takeda – Speakers Bureau. WellTheory – Consultant, Stock Options.
Sang Hee Choi, MD1, Rohit Khanna, DO2, Olivia Lanser, MD2, Luke Irwin, MD3, Thien Nguyen, PharmD4, Mazer Ally, MD5, Rebecca Matro, MD4, Amy Lightner, MD6, Robert Brookover, MD4, Keith Beiermeister, MD7, M. Jonathan Worsey, MD4, Lynn Weston, MD7, Melissa Ferrari, PA-C, MPAS7, Gauree Konijeti, MD, MPH, FACG7. P1131 - Prior Biologic or Janus Kinase Inhibitor Therapy Does Not Impact Colectomy Rates Among Patients Needing Infliximab Rescue Therapy for Acute Severe Ulcerative Colitis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Scripps Green Hospital, San Diego, CA; 2Scripps Green Hospital, La Jolla, CA; 3Scripps Mercy Hospital, San Diego, CA; 4Scripps Health, San Diego, CA; 5Scripps Clinic Medical Group, San Diego, CA; 6Scripps Clinic, La Jolla, CA; 7Scripps Clinic Medical Group, La Jolla, CA
Introduction: Infliximab (IFX) is recommended for steroid-refractory patients hospitalized with acute severe ulcerative colitis (ASUC). However, limited data exist on its efficacy in patients with prior exposure to advanced therapies (AT), including biologics or janus kinase (JAK) inhibitors. This study examines the efficacy of IFX rescue therapy in ASUC patients with and without prior AT exposure.
Methods: We conducted a health system-wide retrospective cohort study of hospitalized patients aged ≥17 years who received inpatient IFX rescue therapy for ASUC between January 2017 and December 2024. The primary endpoint was colectomy rates among AT-naïve vs. AT-exposed patients during index hospitalization and within 3 months following inpatient IFX treatment. Secondary endpoints included the impact of inpatient IFX dosing strategy (standard vs. accelerated), post-discharge IFX persistence at 1 year, use of other AT during follow-up, and colectomy rate at 1 year.
Results: A total of 75 patients received IFX rescue therapy (AT-naïve = 52, AT-exposed = 23). Among AT-exposed, 65% had received 1 prior therapy, 30% had received 2, and 4% had received ≥ 3; 16 patients (28%) were actively on AT at the time of index hospitalization (Table 1). UC duration, CRP, and albumin levels on admission did not differ between groups. AT-naïve patients were more likely to have a Mayo endoscopic subscore of 3 (87% vs. 65% AT-exposed, p=0.03). No significant differences were observed for inpatient (p >0.99) or 3-month (p=0.16) colectomy rates between AT-naïve and AT-exposed patients (Figure 1). Infliximab dosing strategy, whether standard or accelerated (increased frequency, dosing, or both), did not differ between groups and was not independently associated with the primary outcome. AT-exposed patients were more likely to continue IFX at 1 year (52% vs 11%, p< 0.01). Colectomy rates at 1 year were similar (21.7% for AT-exposed vs. 21.2% for AT-naïve, p >0.99).
Discussion: Prior exposure to biologic or JAK inhibitors does not appear to impact short-term colectomy rates following IFX rescue therapy for ASUC, suggesting that IFX remains an effective salvage option regardless of prior treatment history. Future studies are needed to evaluate long-term clinical outcomes.

Figure: Table 1. Baseline demographic and clinical characteristics of study cohort.

Figure: Figure 1. Colectomy rates during index hospitalization and up to 3 months in advance therapy naïve vs. exposed patients.
Disclosures:
Sang Hee Choi indicated no relevant financial relationships.
Rohit Khanna indicated no relevant financial relationships.
Olivia Lanser indicated no relevant financial relationships.
Luke Irwin indicated no relevant financial relationships.
Thien Nguyen indicated no relevant financial relationships.
Mazer Ally: Abbvie – Speakers Bureau. Lilly – Advisory Committee/Board Member.
Rebecca Matro indicated no relevant financial relationships.
Amy Lightner indicated no relevant financial relationships.
Robert Brookover indicated no relevant financial relationships.
Keith Beiermeister indicated no relevant financial relationships.
M. Jonathan Worsey indicated no relevant financial relationships.
Lynn Weston indicated no relevant financial relationships.
Melissa Ferrari: Abbvie – Speakers Bureau. Eli Lilly – Advisory Committee/Board Member. Johnson and Johnson – Speakers Bureau. Pfizer – Advisory Committee/Board Member.
Gauree Konijeti: Abbvie – Advisory Committee/Board Member, Consultant. Johnson and Johnson – Consultant. Lilly – Consultant, Speakers Bureau. Pfizer – Advisory Committee/Board Member. Takeda – Speakers Bureau. WellTheory – Consultant, Stock Options.
Sang Hee Choi, MD1, Rohit Khanna, DO2, Olivia Lanser, MD2, Luke Irwin, MD3, Thien Nguyen, PharmD4, Mazer Ally, MD5, Rebecca Matro, MD4, Amy Lightner, MD6, Robert Brookover, MD4, Keith Beiermeister, MD7, M. Jonathan Worsey, MD4, Lynn Weston, MD7, Melissa Ferrari, PA-C, MPAS7, Gauree Konijeti, MD, MPH, FACG7. P1131 - Prior Biologic or Janus Kinase Inhibitor Therapy Does Not Impact Colectomy Rates Among Patients Needing Infliximab Rescue Therapy for Acute Severe Ulcerative Colitis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
