Sunday Poster Session
Category: IBD
P1126 - Glucagon-Like Peptide-1 Receptor Agonist Therapy Is Associated With Decreased Risk of Recurrent Pouchitis in Obese Patients With Ulcerative Colitis and IPAA: A Propensity-Matched Cohort Study
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall
.jpg)
Hany Habib, MD
Allegheny Health Network Medicine Institute
Pittsburgh, PA
Presenting Author(s)
Aakash Desai, MD1, Hany Habib, MD2, Himsikhar Khataniar, MD3, Francis A.. Farraye, MD, MSc, MACG4, Priya Sehgal, MD5, Edward L.. Barnes, MD, MPH6, Gursimran Kochhar, MD1, Jana G. Hashash, MD, MSc, FACG4
1Allegheny Health Network, Pittsburgh, PA; 2Allegheny Health Network Medicine Institute, Pittsburgh, PA; 3Allegheny General Hospital, Pittsburgh, PA; 4Mayo Clinic, Jacksonville, FL; 5Thomas Jefferson University Hospital, Philadelphia, PA; 6University of North Carolina at Chapel Hill, Chapel Hill, NC
Introduction: Obesity is associated with increased pouchitis risk, potentially contributing to chronic intestinal inflammation. Glucagon-like peptide-1 receptor agonists (GLP-1RAs) promote weight loss, improve glycemic control, and exhibit anti-inflammatory properties beneficial in inflammatory bowel disease (IBD). However, their role in recurrent pouchitis remains unclear.
Methods: We conducted a retrospective cohort study using the TriNetX database to evaluate the risk of recurrent pouchitis in a cohort of patients with obesity and ileal pouch-anal anastomosis (IPAA) with pouchitis history who initiated GLP-1RA compared to those who did not. The primary outcome was risk of repeat antibiotic use (ciprofloxacin and/or metronidazole) after 30 days and within 1 year. Validated case definitions of IPAA and recurrent pouchitis were used. Patients with advanced therapy (AT) use before the index event and after IPAA were excluded. Secondary outcomes included number of antibiotic prescriptions, AT use, anti-diarrheal use, Crohn’s like disease of pouch (CLDP), pouch excision and healthcare utilization. 1:1 propensity score matching was performed.
Results: The GLP-1RA cohort included 43 patients (mean age 51.2 ± 14.7; BMI 35.1 ± 6.4 kg/m2; 62.8% female; 72% White; 44.1% T2DM), matched to 509 control patients. 53.4% had pouchitis and 34.8% had ≥ 2 episodes in the preceding 1 year in the GLP-1RA cohort. GLP-1RA cohort had a significantly decreased risk of recurrent pouchitis (26.3% vs. 52.6%; aHR 0.45, 95% CI 0.21-0.96; log rank p=0.03) compared to the control cohort. Mean antibiotic prescriptions were numerically lower in the GLP-1RA cohort but not statistically significant (2 vs 4.4, p=0.16). There was no difference in the risk of AT use or CLDP and no patients required pouch excision. Sensitivity analysis excluding antibiotic prescriptions within 1 and 3 months in the control cohort also showed a decreased risk of recurrent pouchitis in the GLP-1RA cohort. Anti-diarrheal use was lower in the GLP1-RA cohort. Within the GLP-1RA cohort, BMI significantly decreased by 3.2 kg/m² within 12 months (95% CI −5.8 to −0.50; p=0.03). The mean weight loss was 16 lbs (-39.5 to -6.5). Rates of serious adverse events were low in the GLP-1RA cohort.
Discussion: In patients with obesity and IPAA with history of pouchitis, GLP-1RA use was associated with decrease in pouchitis recurrence, BMI, and body weight, suggesting beneficial effects on pouch inflammation.
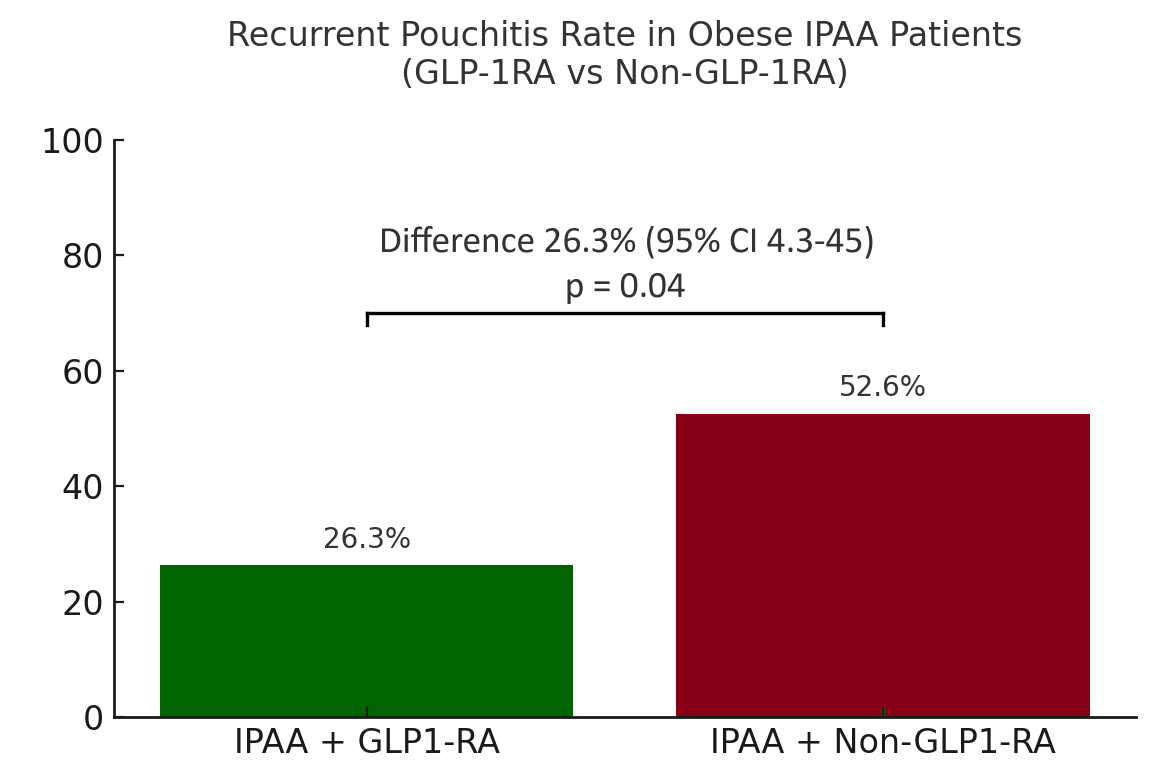
Figure: Figure 1: Risk of recurrent pouchitis in UC-IPAA patients with obesity in the GLP-1RA and non-GLP-1RA cohort.
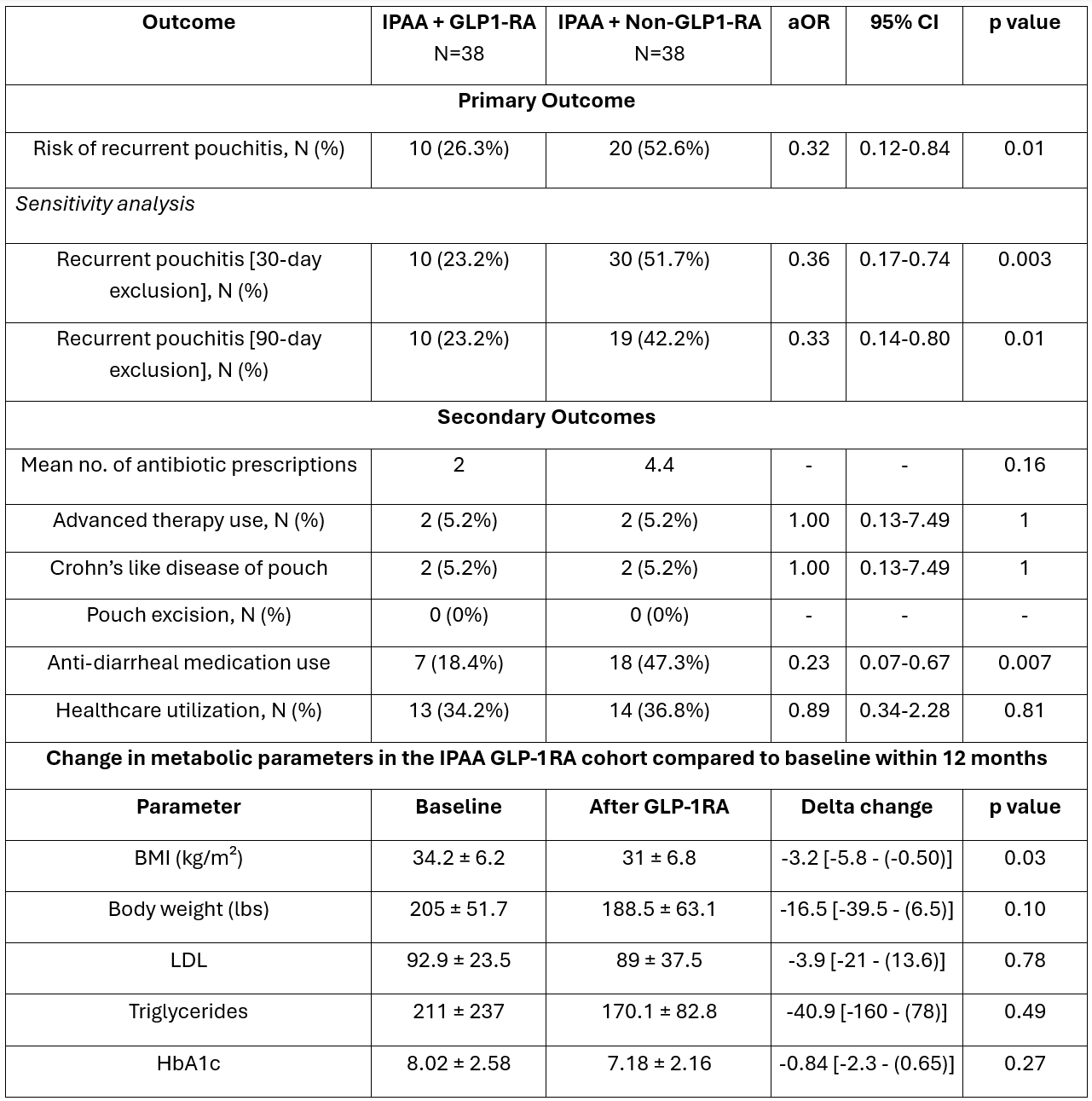
Figure: Table 1. Comparison of outcomes between obese IPAA GLP-1RA cohort and obese IPAA Control cohort after 1:1 propensity score matching within 12 months. Change metabolic parameters in the IPAA GLP-1RA cohort compared to baseline within 12 months. Sensitivity analysis conducted to exclude repeat antibiotic prescriptions within 30 and 90 days from previous pouchitis episode in the control cohort with subsequent 1 year follow up.
Disclosures:
Aakash Desai indicated no relevant financial relationships.
Hany Habib indicated no relevant financial relationships.
Himsikhar Khataniar indicated no relevant financial relationships.
Francis Farraye: Astellas – Advisory Committee/Board Member. Avalo – Advisory Committee/Board Member. Bausch – Advisory Committee/Board Member. BMS – Advisory Committee/Board Member. Braintree Labs – Advisory Committee/Board Member. Fresenius Kabi – Advisory Committee/Board Member. GI Reviewers – Independent Contractor. IBD Educational Group – Independent Contractor. Iterative Health – Advisory Committee/Board Member, Stock Options. Janssen – Advisory Committee/Board Member. Lilly – DSMB. Pfizer – Advisory Committee/Board Member. Pharmacosmos – Advisory Committee/Board Member. Sandoz – Advisory Committee/Board Member. Viatris – Advisory Committee/Board Member.
Priya Sehgal indicated no relevant financial relationships.
Edward Barnes: AbbVie, Inc. – Consultant. Boomerang – Consultant. Eli Lilly – Consultant. Pfizer – Consultant. Sanofi – Consultant. Takeda – Consultant. Target RWE – Consultant.
Gursimran Kochhar indicated no relevant financial relationships.
Jana Hashash: BMS – Ad Board.
Aakash Desai, MD1, Hany Habib, MD2, Himsikhar Khataniar, MD3, Francis A.. Farraye, MD, MSc, MACG4, Priya Sehgal, MD5, Edward L.. Barnes, MD, MPH6, Gursimran Kochhar, MD1, Jana G. Hashash, MD, MSc, FACG4. P1126 - Glucagon-Like Peptide-1 Receptor Agonist Therapy Is Associated With Decreased Risk of Recurrent Pouchitis in Obese Patients With Ulcerative Colitis and IPAA: A Propensity-Matched Cohort Study, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Allegheny Health Network, Pittsburgh, PA; 2Allegheny Health Network Medicine Institute, Pittsburgh, PA; 3Allegheny General Hospital, Pittsburgh, PA; 4Mayo Clinic, Jacksonville, FL; 5Thomas Jefferson University Hospital, Philadelphia, PA; 6University of North Carolina at Chapel Hill, Chapel Hill, NC
Introduction: Obesity is associated with increased pouchitis risk, potentially contributing to chronic intestinal inflammation. Glucagon-like peptide-1 receptor agonists (GLP-1RAs) promote weight loss, improve glycemic control, and exhibit anti-inflammatory properties beneficial in inflammatory bowel disease (IBD). However, their role in recurrent pouchitis remains unclear.
Methods: We conducted a retrospective cohort study using the TriNetX database to evaluate the risk of recurrent pouchitis in a cohort of patients with obesity and ileal pouch-anal anastomosis (IPAA) with pouchitis history who initiated GLP-1RA compared to those who did not. The primary outcome was risk of repeat antibiotic use (ciprofloxacin and/or metronidazole) after 30 days and within 1 year. Validated case definitions of IPAA and recurrent pouchitis were used. Patients with advanced therapy (AT) use before the index event and after IPAA were excluded. Secondary outcomes included number of antibiotic prescriptions, AT use, anti-diarrheal use, Crohn’s like disease of pouch (CLDP), pouch excision and healthcare utilization. 1:1 propensity score matching was performed.
Results: The GLP-1RA cohort included 43 patients (mean age 51.2 ± 14.7; BMI 35.1 ± 6.4 kg/m2; 62.8% female; 72% White; 44.1% T2DM), matched to 509 control patients. 53.4% had pouchitis and 34.8% had ≥ 2 episodes in the preceding 1 year in the GLP-1RA cohort. GLP-1RA cohort had a significantly decreased risk of recurrent pouchitis (26.3% vs. 52.6%; aHR 0.45, 95% CI 0.21-0.96; log rank p=0.03) compared to the control cohort. Mean antibiotic prescriptions were numerically lower in the GLP-1RA cohort but not statistically significant (2 vs 4.4, p=0.16). There was no difference in the risk of AT use or CLDP and no patients required pouch excision. Sensitivity analysis excluding antibiotic prescriptions within 1 and 3 months in the control cohort also showed a decreased risk of recurrent pouchitis in the GLP-1RA cohort. Anti-diarrheal use was lower in the GLP1-RA cohort. Within the GLP-1RA cohort, BMI significantly decreased by 3.2 kg/m² within 12 months (95% CI −5.8 to −0.50; p=0.03). The mean weight loss was 16 lbs (-39.5 to -6.5). Rates of serious adverse events were low in the GLP-1RA cohort.
Discussion: In patients with obesity and IPAA with history of pouchitis, GLP-1RA use was associated with decrease in pouchitis recurrence, BMI, and body weight, suggesting beneficial effects on pouch inflammation.

Figure: Figure 1: Risk of recurrent pouchitis in UC-IPAA patients with obesity in the GLP-1RA and non-GLP-1RA cohort.

Figure: Table 1. Comparison of outcomes between obese IPAA GLP-1RA cohort and obese IPAA Control cohort after 1:1 propensity score matching within 12 months. Change metabolic parameters in the IPAA GLP-1RA cohort compared to baseline within 12 months. Sensitivity analysis conducted to exclude repeat antibiotic prescriptions within 30 and 90 days from previous pouchitis episode in the control cohort with subsequent 1 year follow up.
Disclosures:
Aakash Desai indicated no relevant financial relationships.
Hany Habib indicated no relevant financial relationships.
Himsikhar Khataniar indicated no relevant financial relationships.
Francis Farraye: Astellas – Advisory Committee/Board Member. Avalo – Advisory Committee/Board Member. Bausch – Advisory Committee/Board Member. BMS – Advisory Committee/Board Member. Braintree Labs – Advisory Committee/Board Member. Fresenius Kabi – Advisory Committee/Board Member. GI Reviewers – Independent Contractor. IBD Educational Group – Independent Contractor. Iterative Health – Advisory Committee/Board Member, Stock Options. Janssen – Advisory Committee/Board Member. Lilly – DSMB. Pfizer – Advisory Committee/Board Member. Pharmacosmos – Advisory Committee/Board Member. Sandoz – Advisory Committee/Board Member. Viatris – Advisory Committee/Board Member.
Priya Sehgal indicated no relevant financial relationships.
Edward Barnes: AbbVie, Inc. – Consultant. Boomerang – Consultant. Eli Lilly – Consultant. Pfizer – Consultant. Sanofi – Consultant. Takeda – Consultant. Target RWE – Consultant.
Gursimran Kochhar indicated no relevant financial relationships.
Jana Hashash: BMS – Ad Board.
Aakash Desai, MD1, Hany Habib, MD2, Himsikhar Khataniar, MD3, Francis A.. Farraye, MD, MSc, MACG4, Priya Sehgal, MD5, Edward L.. Barnes, MD, MPH6, Gursimran Kochhar, MD1, Jana G. Hashash, MD, MSc, FACG4. P1126 - Glucagon-Like Peptide-1 Receptor Agonist Therapy Is Associated With Decreased Risk of Recurrent Pouchitis in Obese Patients With Ulcerative Colitis and IPAA: A Propensity-Matched Cohort Study, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.

