Sunday Poster Session
Category: IBD
Clinical Outcomes of Fidaxomicin vs Vancomycin in Treating <i>Clostridioides difficile</i> Infections in Patients With Inflammatory Bowel Disease
P1108 - Clinical Outcomes of Fidaxomicin vs Vancomycin in Treating Clostridioides difficile Infections in Patients With Inflammatory Bowel Disease
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall
- UU
Ugo Uguru, MD (she/her/hers)
NYU Langone Health
New York, NY
Presenting Author(s)
Ugo Uguru, MD1, Larry Nguyen, MD1, Olivia Delau, MS1, Adam Faye, MD, MS1, Jordan Axelrad, MD, MPH2
1NYU Langone Health, New York, NY; 2Division of Gastroenterology, Department of Medicine, NYU Grossman School of Medicine, New York, NY
Introduction: Patients with inflammatory bowel disease (IBD) are particularly vulnerable to Clostridioides difficile infection (CDI). Recent society guidelines recommend fidaxomicin as the preferred standard-of-care (SOC) treatment for CDI over vancomycin. This study aims to compare the efficacy of treatment and clinical outcomes of IBD patients with CDI following SOC treatment with either vancomycin or fidaxomicin.
Methods: We identified adult patients with IBD [Ulcerative Colitis (UC) or Crohn’s Disease (CD)] with new or recurrent CDI who were treated with either vancomycin or fidaxomicin at an academic medical center between January 2022 to December 2024. Baseline demographics, IBD clinical characteristics, comorbidities, and prior CDI history were obtained. Cox proportional hazard analysis assessing time to a composite of CDI recurrence and IBD severity outcomes within 1 year from end of CDI treatment was performed. IBD severity outcomes included change in IBD therapy, new corticosteroid therapy, IBD surgery, IBD-related hospitalization, IBD-related ER visits, toxic megacolon, colon perforation, and septic shock.
Results: We identified 182 patients with IBD whose CDI was treated with either vancomycin (n=136) or fidaxomicin (n=46). Patient characteristics were similar across treatment groups except prior CDI infections were higher in fidaxomicin compared to vancomycin (50.0% vs 29.4%, p=0.01) and the length of CDI treatment was shorter in fidaxomicin compared to vancomycin group (median 11 vs 23 days, p< 0.01; Table 1). CDI recurrence within 1-year among all IBD patients was 3.9%, with 2.2% and 4.4% (p = 0.68) in groups treated with fidaxomicin and vancomycin, respectively. IBD patients treated with fidaxomicin had numerically fewer outcomes related to new corticosteroid therapy, IBD surgery, IBD related hospitalization and ER visits compared to patients treated with vancomycin but were not statistically significant (Table 2). There were no significant associations between type of SOC therapy (aHR = 1.47; p = 0.19) or hospitalization due to CDI (aHR = 1.64; p=0.05) and risk of 1-year CDI recurrence and IBD severity outcomes (p = 0.15).
Discussion: Vancomycin and fidaxomicin are efficient in treating CDI in patients with IBD. Fidaxomicin was associated with numerically lower recurrence and adverse IBD outcomes, however, this association was not significant after adjusting for IBD and CDI confounders. Further studies with large cohorts are needed to verify these findings.
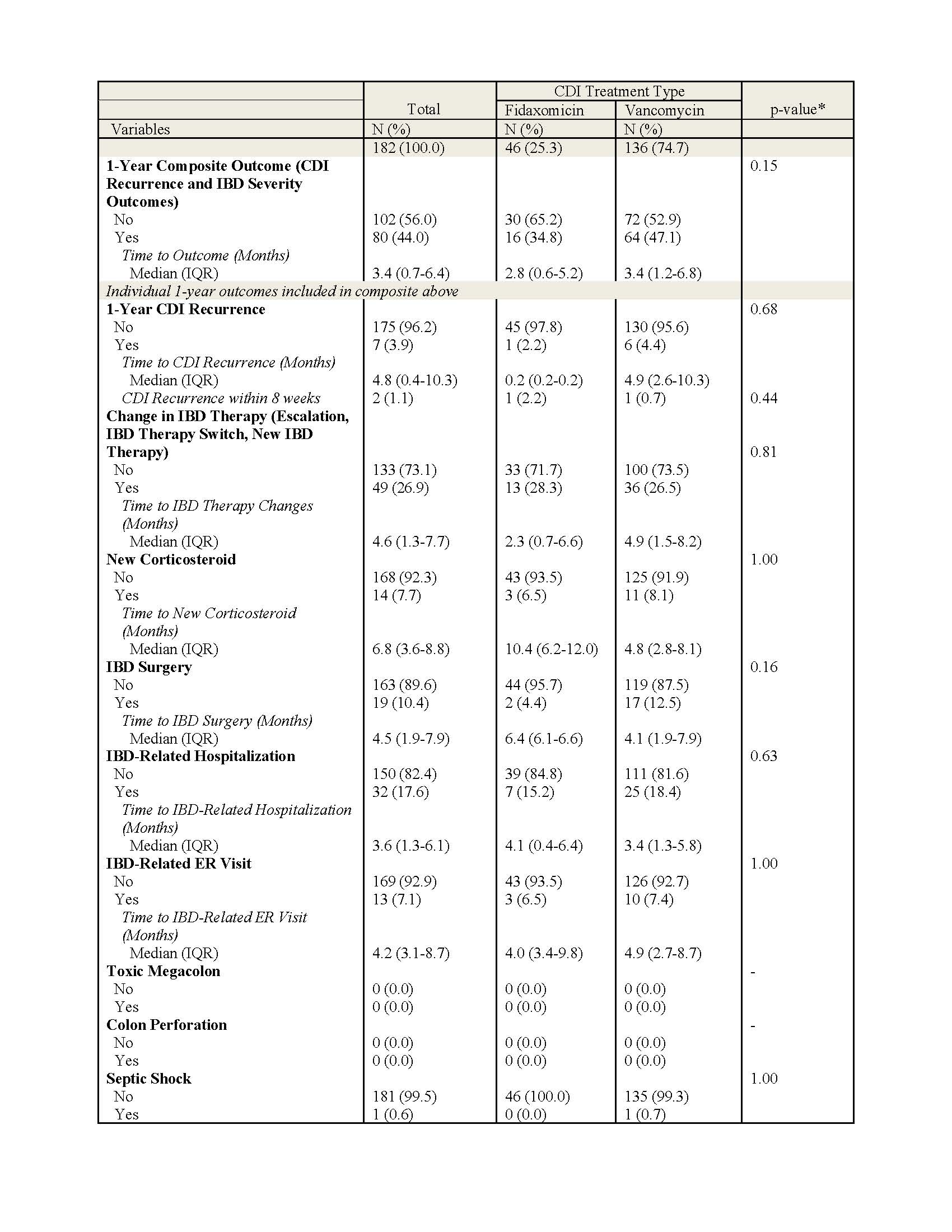
Figure: Table 1. Baseline Demographic & Clinical Characteristics by CDI Treatment, N=182.
Based on SES-CD & UC Mayo scores from most recent colonoscopy prior to CDI diagnosis (within 1 year prior)
*P-values calculated by Wilcoxon Rank-Sum tests for continuous variables and Pearson’s Chi Squared or Fisher’s Exact tests for categorical variables. Bolded values indicate statistical significance (p < 0.05)
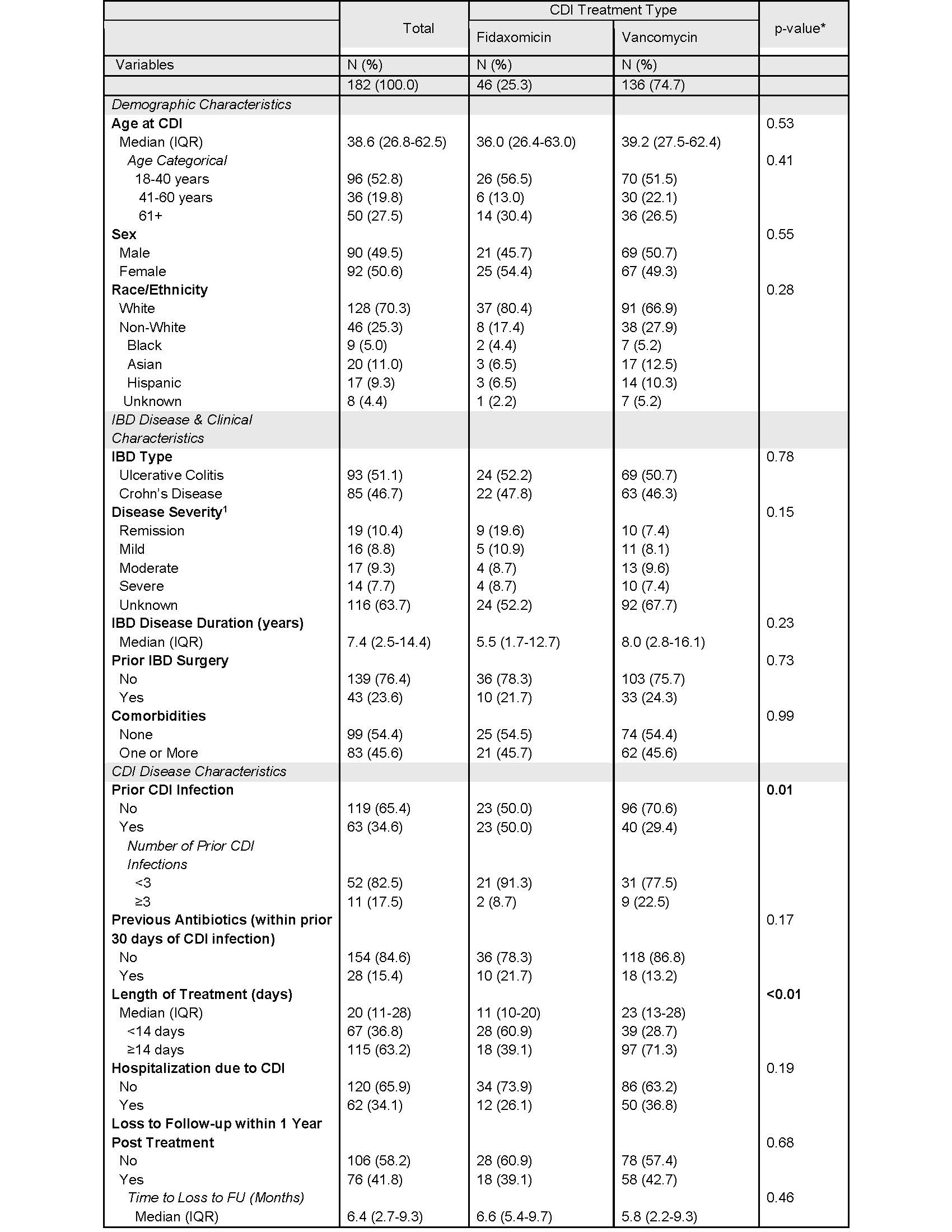
Figure: Table 2. 1-Year Outcomes Stratified by Treatment Type.
Primary outcome is composite of CDI recurrence and IBD severity outcomes within 1 year from end of CDI treatment. IBD severity outcomes include change in IBD therapy (escalation, IBD therapy switch, new IBD therapy), new corticosteroid prescription, IBD surgery, IBD-related hospitalization, IBD-
related ER visit, toxic megacolon, colon perforation, septic shock. Patients were followed from end of treatment to outcome, last follow-up date, or 1 year post treatment (whichever came first).
Disclosures:
Ugo Uguru indicated no relevant financial relationships.
Larry Nguyen indicated no relevant financial relationships.
Olivia Delau indicated no relevant financial relationships.
Adam Faye: AbbVie – Honorarium. Eli Lilly – Consultant. NIH Grant K76AG083286 – Grant/Research Support. Takeda – Honorarium. The American College of Gastroenterology – Grant/Research Support. The Crohn's and Colitis Foundation – Grant/Research Support.
Jordan Axelrad: Abbvie – Advisory Committee/Board Member, Consultant, Honorarium. Abivax – Advisory Committee/Board Member, Consultant, Honorarium. Adiso – Advisory Committee/Board Member, Consultant, Honorarium. BioFire Diagnostics – Grant/Research Support. Biomerieux – Advisory Committee/Board Member, Consultant, Honorarium. Bristol-Myers Squibb – Advisory Committee/Board Member, Consultant, Honorarium. Celltrion – Advisory Committee/Board Member, Consultant, Honorarium. Ferring – Advisory Committee/Board Member, Consultant, Honorarium. Fresenius – Advisory Committee/Board Member, Consultant, Honorarium. Genentech – Grant/Research Support. Janssen – Advisory Committee/Board Member, Consultant. Janssen – Grant/Research Support. Janssen – Honorarium. Johnson & Johnson – Advisory Committee/Board Member, Consultant. Merck – Advisory Committee/Board Member, Consultant, Honorarium. NIH NIDDK Diseases K23DK124570 – Grant/Research Support. Pfizer – Advisory Committee/Board Member, Consultant, Honorarium. Sanofi – Advisory Committee/Board Member, Consultant, Honorarium. The Crohn's and Colitis Foundation (#878246) – Grant/Research Support. The Judith & Stewart Colton Center for Autoimmunity – Grant/Research Support. Vedanta – Advisory Committee/Board Member, Consultant, Honorarium.
Ugo Uguru, MD1, Larry Nguyen, MD1, Olivia Delau, MS1, Adam Faye, MD, MS1, Jordan Axelrad, MD, MPH2. P1108 - Clinical Outcomes of Fidaxomicin vs Vancomycin in Treating <i>Clostridioides difficile</i> Infections in Patients With Inflammatory Bowel Disease, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1NYU Langone Health, New York, NY; 2Division of Gastroenterology, Department of Medicine, NYU Grossman School of Medicine, New York, NY
Introduction: Patients with inflammatory bowel disease (IBD) are particularly vulnerable to Clostridioides difficile infection (CDI). Recent society guidelines recommend fidaxomicin as the preferred standard-of-care (SOC) treatment for CDI over vancomycin. This study aims to compare the efficacy of treatment and clinical outcomes of IBD patients with CDI following SOC treatment with either vancomycin or fidaxomicin.
Methods: We identified adult patients with IBD [Ulcerative Colitis (UC) or Crohn’s Disease (CD)] with new or recurrent CDI who were treated with either vancomycin or fidaxomicin at an academic medical center between January 2022 to December 2024. Baseline demographics, IBD clinical characteristics, comorbidities, and prior CDI history were obtained. Cox proportional hazard analysis assessing time to a composite of CDI recurrence and IBD severity outcomes within 1 year from end of CDI treatment was performed. IBD severity outcomes included change in IBD therapy, new corticosteroid therapy, IBD surgery, IBD-related hospitalization, IBD-related ER visits, toxic megacolon, colon perforation, and septic shock.
Results: We identified 182 patients with IBD whose CDI was treated with either vancomycin (n=136) or fidaxomicin (n=46). Patient characteristics were similar across treatment groups except prior CDI infections were higher in fidaxomicin compared to vancomycin (50.0% vs 29.4%, p=0.01) and the length of CDI treatment was shorter in fidaxomicin compared to vancomycin group (median 11 vs 23 days, p< 0.01; Table 1). CDI recurrence within 1-year among all IBD patients was 3.9%, with 2.2% and 4.4% (p = 0.68) in groups treated with fidaxomicin and vancomycin, respectively. IBD patients treated with fidaxomicin had numerically fewer outcomes related to new corticosteroid therapy, IBD surgery, IBD related hospitalization and ER visits compared to patients treated with vancomycin but were not statistically significant (Table 2). There were no significant associations between type of SOC therapy (aHR = 1.47; p = 0.19) or hospitalization due to CDI (aHR = 1.64; p=0.05) and risk of 1-year CDI recurrence and IBD severity outcomes (p = 0.15).
Discussion: Vancomycin and fidaxomicin are efficient in treating CDI in patients with IBD. Fidaxomicin was associated with numerically lower recurrence and adverse IBD outcomes, however, this association was not significant after adjusting for IBD and CDI confounders. Further studies with large cohorts are needed to verify these findings.

Figure: Table 1. Baseline Demographic & Clinical Characteristics by CDI Treatment, N=182.
Based on SES-CD & UC Mayo scores from most recent colonoscopy prior to CDI diagnosis (within 1 year prior)
*P-values calculated by Wilcoxon Rank-Sum tests for continuous variables and Pearson’s Chi Squared or Fisher’s Exact tests for categorical variables. Bolded values indicate statistical significance (p < 0.05)

Figure: Table 2. 1-Year Outcomes Stratified by Treatment Type.
Primary outcome is composite of CDI recurrence and IBD severity outcomes within 1 year from end of CDI treatment. IBD severity outcomes include change in IBD therapy (escalation, IBD therapy switch, new IBD therapy), new corticosteroid prescription, IBD surgery, IBD-related hospitalization, IBD-
related ER visit, toxic megacolon, colon perforation, septic shock. Patients were followed from end of treatment to outcome, last follow-up date, or 1 year post treatment (whichever came first).
Disclosures:
Ugo Uguru indicated no relevant financial relationships.
Larry Nguyen indicated no relevant financial relationships.
Olivia Delau indicated no relevant financial relationships.
Adam Faye: AbbVie – Honorarium. Eli Lilly – Consultant. NIH Grant K76AG083286 – Grant/Research Support. Takeda – Honorarium. The American College of Gastroenterology – Grant/Research Support. The Crohn's and Colitis Foundation – Grant/Research Support.
Jordan Axelrad: Abbvie – Advisory Committee/Board Member, Consultant, Honorarium. Abivax – Advisory Committee/Board Member, Consultant, Honorarium. Adiso – Advisory Committee/Board Member, Consultant, Honorarium. BioFire Diagnostics – Grant/Research Support. Biomerieux – Advisory Committee/Board Member, Consultant, Honorarium. Bristol-Myers Squibb – Advisory Committee/Board Member, Consultant, Honorarium. Celltrion – Advisory Committee/Board Member, Consultant, Honorarium. Ferring – Advisory Committee/Board Member, Consultant, Honorarium. Fresenius – Advisory Committee/Board Member, Consultant, Honorarium. Genentech – Grant/Research Support. Janssen – Advisory Committee/Board Member, Consultant. Janssen – Grant/Research Support. Janssen – Honorarium. Johnson & Johnson – Advisory Committee/Board Member, Consultant. Merck – Advisory Committee/Board Member, Consultant, Honorarium. NIH NIDDK Diseases K23DK124570 – Grant/Research Support. Pfizer – Advisory Committee/Board Member, Consultant, Honorarium. Sanofi – Advisory Committee/Board Member, Consultant, Honorarium. The Crohn's and Colitis Foundation (#878246) – Grant/Research Support. The Judith & Stewart Colton Center for Autoimmunity – Grant/Research Support. Vedanta – Advisory Committee/Board Member, Consultant, Honorarium.
Ugo Uguru, MD1, Larry Nguyen, MD1, Olivia Delau, MS1, Adam Faye, MD, MS1, Jordan Axelrad, MD, MPH2. P1108 - Clinical Outcomes of Fidaxomicin vs Vancomycin in Treating <i>Clostridioides difficile</i> Infections in Patients With Inflammatory Bowel Disease, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
