Sunday Poster Session
Category: IBD
P1106 - Intravenous versus Subcutaneous Infliximab for Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall
- OA
Omar Al-Radideh, MD
University of Florida College of Medicine
Gainesville, FL
Presenting Author(s)
Muhammad Ahsan Asif, MBBS1, Azeem Khalid, MD2, Mudasar Nisar, 3, Rabia Javed, 3, Ahmed Raza, 3, Omar Al-Radideh, MD4, Adnan Bhat, MD5
1Jinnah Hospital Lahore, Lahore, Punjab, Pakistan; 2Aiken Regional Medical Centers, Aiken, SC; 3Services Institute of Medical Sciences, Lahore, Punjab, Pakistan; 4University of Florida College of Medicine, Gainesville, FL; 5University of Florida, Gainesville, FL
Introduction: Infliximab, a tumor necrosis factor-alpha (TNF-α) inhibitor, is pivotal in managing inflammatory bowel disease (IBD), including Crohn’s disease (CD) and ulcerative colitis (UC). While the intravenous (IV) formulation has been standard, a subcutaneous (SC) form was recently approved, potentially offering greater convenience and healthcare efficiency. This meta-analysis compares the efficacy and safety of IV versus SC infliximab in IBD.
Methods: A systematic search of PubMed and Scopus (through March 2025) identified randomized trials, cohorts, and observational studies involving adults with UC, CD, or IBD. Meta-analysis was conducted using R software (version 4.1.2). Relative risk (RR) and weighted mean difference (WMD) with 95% confidence intervals (CI) were used to assess dichotomous and continuous outcomes. Forest and funnel plots were generated. Heterogeneity was assessed using I² statistics. Risk of bias was evaluated with the Cochrane ROB 2 tool.
Results: Seventeen studies (n = 2,885) met inclusion criteria. Pooled analysis showed significantly higher remission rates with IV infliximab (RR = 0.90; 95% CI: 0.83–0.97; I² = 68%; p < 0.01). Subgroup analysis by disease type (UC, CD, or IBD) revealed no significant effect modification (p = 0.48). Stratification by follow-up period showed a statistically significant difference favoring IV at 6 months. Injection-site reactions occurred in 24% of SC users (95% CI: 15%–34%), while infusion reactions occurred in 46% of IV users (95% CI: 36%–57%; I² = 0). Serious adverse events were reported in 23% of SC recipients (95% CI: 0%–48%), but inconsistent safety reporting limited comparisons.
These findings contrast with a prior meta-analysis (Chetwood et al., J Crohn’s Colitis, 2024), which found no loss of remission after switching stable patients from IV to SC. Our broader analysis suggests IV may be more effective in achieving or maintaining remission
Discussion: This meta-analysis indicates that IV infliximab is associated with significant improvement in remission rates compared to the SC form in IBD patients. Safety profiles differ, but inconsistent adverse event reporting limits definitive conclusions. Standardized efficacy and safety endpoints are essential in future comparative trials to guide optimal route selection.
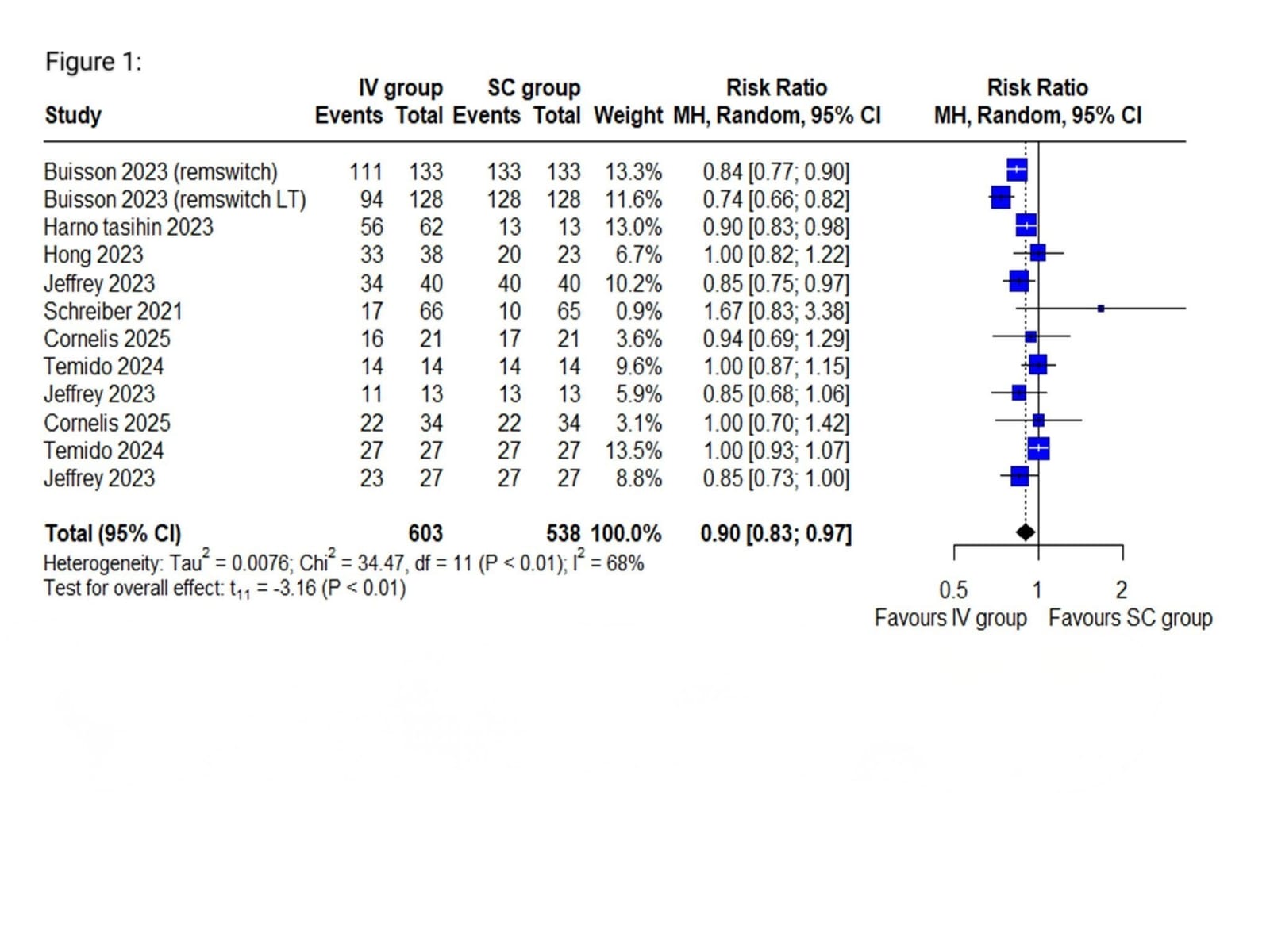
Figure: Figure 1: Forest plot of overall remission rate in SC vs IV group in IBD patients
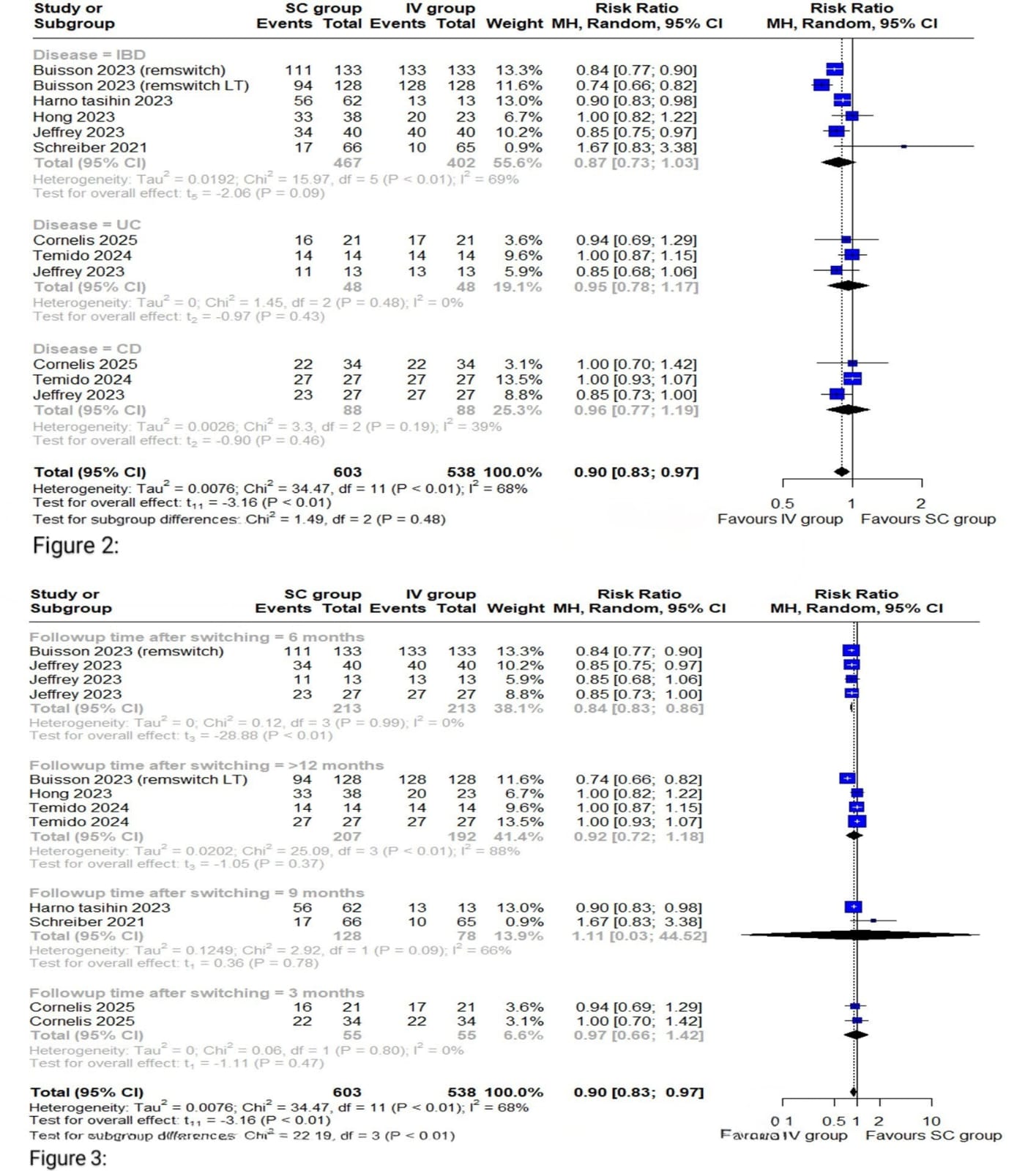
Figure: Figure 2: Forest plot of overall remission rates based on subgroup by Disease type
Figure 3: Forest plot of overall remission rates based on subgroup by follow-up time
Disclosures:
Muhammad Ahsan Asif indicated no relevant financial relationships.
Azeem Khalid indicated no relevant financial relationships.
Mudasar Nisar indicated no relevant financial relationships.
Rabia Javed indicated no relevant financial relationships.
Ahmed Raza indicated no relevant financial relationships.
Omar Al-Radideh indicated no relevant financial relationships.
Adnan Bhat indicated no relevant financial relationships.
Muhammad Ahsan Asif, MBBS1, Azeem Khalid, MD2, Mudasar Nisar, 3, Rabia Javed, 3, Ahmed Raza, 3, Omar Al-Radideh, MD4, Adnan Bhat, MD5. P1106 - Intravenous versus Subcutaneous Infliximab for Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Jinnah Hospital Lahore, Lahore, Punjab, Pakistan; 2Aiken Regional Medical Centers, Aiken, SC; 3Services Institute of Medical Sciences, Lahore, Punjab, Pakistan; 4University of Florida College of Medicine, Gainesville, FL; 5University of Florida, Gainesville, FL
Introduction: Infliximab, a tumor necrosis factor-alpha (TNF-α) inhibitor, is pivotal in managing inflammatory bowel disease (IBD), including Crohn’s disease (CD) and ulcerative colitis (UC). While the intravenous (IV) formulation has been standard, a subcutaneous (SC) form was recently approved, potentially offering greater convenience and healthcare efficiency. This meta-analysis compares the efficacy and safety of IV versus SC infliximab in IBD.
Methods: A systematic search of PubMed and Scopus (through March 2025) identified randomized trials, cohorts, and observational studies involving adults with UC, CD, or IBD. Meta-analysis was conducted using R software (version 4.1.2). Relative risk (RR) and weighted mean difference (WMD) with 95% confidence intervals (CI) were used to assess dichotomous and continuous outcomes. Forest and funnel plots were generated. Heterogeneity was assessed using I² statistics. Risk of bias was evaluated with the Cochrane ROB 2 tool.
Results: Seventeen studies (n = 2,885) met inclusion criteria. Pooled analysis showed significantly higher remission rates with IV infliximab (RR = 0.90; 95% CI: 0.83–0.97; I² = 68%; p < 0.01). Subgroup analysis by disease type (UC, CD, or IBD) revealed no significant effect modification (p = 0.48). Stratification by follow-up period showed a statistically significant difference favoring IV at 6 months. Injection-site reactions occurred in 24% of SC users (95% CI: 15%–34%), while infusion reactions occurred in 46% of IV users (95% CI: 36%–57%; I² = 0). Serious adverse events were reported in 23% of SC recipients (95% CI: 0%–48%), but inconsistent safety reporting limited comparisons.
These findings contrast with a prior meta-analysis (Chetwood et al., J Crohn’s Colitis, 2024), which found no loss of remission after switching stable patients from IV to SC. Our broader analysis suggests IV may be more effective in achieving or maintaining remission
Discussion: This meta-analysis indicates that IV infliximab is associated with significant improvement in remission rates compared to the SC form in IBD patients. Safety profiles differ, but inconsistent adverse event reporting limits definitive conclusions. Standardized efficacy and safety endpoints are essential in future comparative trials to guide optimal route selection.

Figure: Figure 1: Forest plot of overall remission rate in SC vs IV group in IBD patients

Figure: Figure 2: Forest plot of overall remission rates based on subgroup by Disease type
Figure 3: Forest plot of overall remission rates based on subgroup by follow-up time
Disclosures:
Muhammad Ahsan Asif indicated no relevant financial relationships.
Azeem Khalid indicated no relevant financial relationships.
Mudasar Nisar indicated no relevant financial relationships.
Rabia Javed indicated no relevant financial relationships.
Ahmed Raza indicated no relevant financial relationships.
Omar Al-Radideh indicated no relevant financial relationships.
Adnan Bhat indicated no relevant financial relationships.
Muhammad Ahsan Asif, MBBS1, Azeem Khalid, MD2, Mudasar Nisar, 3, Rabia Javed, 3, Ahmed Raza, 3, Omar Al-Radideh, MD4, Adnan Bhat, MD5. P1106 - Intravenous versus Subcutaneous Infliximab for Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
