Sunday Poster Session
Category: IBD
P1076 - Severe Influenza-Related Outcomes in Patients With Inflammatory Bowel Disease on Immunosuppressive Therapy: A Propensity-Matched Cohort Analysis
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall

Mohamed Eldesouki, MD
Saint Michael's Medical Center, New York Medical College
Newark, NJ
Presenting Author(s)
Mohamed Eldesouki, MD1, Ahmed Ibrahim, MD2, Mohammed Y. Youssef, MD3, Mohammad Kloub, MD4, Mona Ahmed, 5, Francis A.. Farraye, MD, MSc, MACG6, Jana G. Hashash, MD, MSc, FACG6
1Saint Michael's Medical Center, New York Medical College, Newark, NJ; 2Medical University of South Carolina, Charleston, SC; 3Hunt Regional Medical Center, Greenville, TX; 4New York Medical College - Saint Michael's Medical Center, Bloomfield, NJ; 5Mansoura University School of Medicine, East Newark, NJ; 6Mayo Clinic, Jacksonville, FL
Introduction: Patients with inflammatory bowel disease (IBD) often require immunosuppressive therapy to induce and maintain remission. However, the impact of recent immunosuppression on the severity of influenza-related complications needs to be quantitated. We aimed to evaluate whether recent immunosuppressive therapy increases the risk of these complications.
Methods: We used the TriNetX Analytics Network, to examine adults (≥18 years) with a diagnosis of Crohn’s disease (K50.x) or ulcerative colitis (K51.x) during the 2022–2023 influenza season. Patients with recent immunosuppressive therapy (within 6 months) were compared to those without, using 1:1 propensity score matching (PSM) to balance baseline characteristics. Outcomes assessed included influenza infection and related complications during the influenza season.
Results: A total of 93,793 patients were identified, including 39,741 in the immunosuppressive group and 44,795 in the non-immunosuppressive IBD group. After PSM, each group had 39,741 patients (mean [SD] age, 50 [18] years; 54% women). The incidence of influenza diagnosis was significantly higher in the immunosuppressive group (7.1% vs 4.5%; HR, 1.51; 95% CI, 1.43–1.60; p< 0.001). Hospitalization rates were also increased (6.8% vs 4.5%; HR, 1.48; 95% CI, 1.38–1.58; p< 0.001), as were influenza-related complications (4.0% vs 2.5%; HR, 1.51; 95% CI, 1.39–1.63; p< 0.001). ICU admissions (2.4% vs 1.6%; HR, 1.38; 95% CI, 1.25–1.52; p< 0.001) and mechanical ventilation (0.7% vs 0.5%; HR, 1.32; 95% CI, 1.10–1.59; p=0.003) were both more frequent in the immunosuppressive group. Use of antiviral medications was higher among those in the immunosuppressive cohort (0.8% vs 0.3%; HR, 2.24; 95% CI, 1.82–2.76; p< 0.001), and overall mortality was modestly increased (1.1% vs 0.9%; HR, 1.17; 95% CI, 1.02–1.35; p=0.001). Subgroup analysis revealed the highest risks among patients on corticosteroids (HR 1.62, 95% CI 1.53–1.72), followed by JAK inhibitors (HR 1.53, 95% CI 1.43–1.63), and anti-IL-23 agents (HR 1.53, 95% CI 1.43–1.63). Anti-IL-12/23 and anti-TNF agents were also associated with elevated risks (Figure).
Discussion: Patients with IBD requiring immunosuppressive therapy had a significantly higher risk of influenza infection and severe complications compared to non-immunosuppressed counterparts. These findings highlight the importance of annual influenza vaccination and timely antiviral treatment in patients with IBD, particularly those on immunosuppressive therapy.
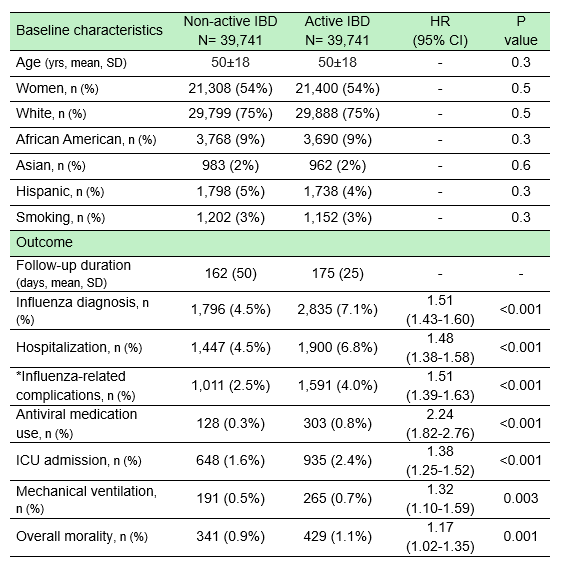
Figure: Table: Clinical features and outcomes
Abbreviations: IBD: inflammatory bowel disease; ICD: intensive care unit; SD: standard deviation; HR: hazard ratio.
*This included pneumonia, sepsis, acute bronchitis, myocarditis, and encephalopathy.
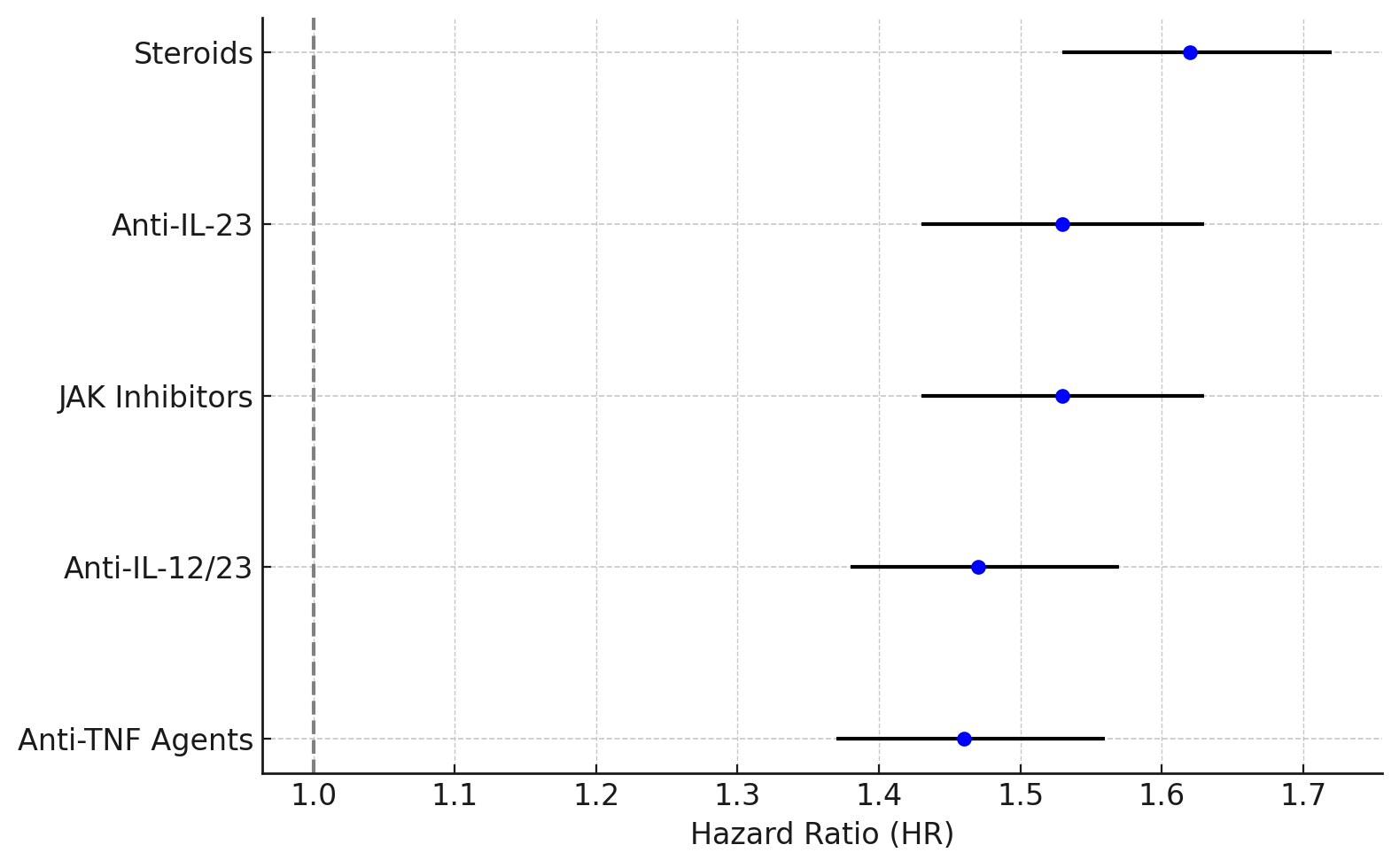
Figure: Figure. Forest plot depicting the risk of influenza infection in different immunosuppressive medications.
Disclosures:
Mohamed Eldesouki indicated no relevant financial relationships.
Ahmed Ibrahim indicated no relevant financial relationships.
Mohammed Y. Youssef indicated no relevant financial relationships.
Mohammad Kloub indicated no relevant financial relationships.
Mona Ahmed indicated no relevant financial relationships.
Francis Farraye: Astellas – Advisory Committee/Board Member. Avalo – Advisory Committee/Board Member. Bausch – Advisory Committee/Board Member. BMS – Advisory Committee/Board Member. Braintree Labs – Advisory Committee/Board Member. Fresenius Kabi – Advisory Committee/Board Member. GI Reviewers – Independent Contractor. IBD Educational Group – Independent Contractor. Iterative Health – Advisory Committee/Board Member, Stock Options. Janssen – Advisory Committee/Board Member. Lilly – DSMB. Pfizer – Advisory Committee/Board Member. Pharmacosmos – Advisory Committee/Board Member. Sandoz – Advisory Committee/Board Member. Viatris – Advisory Committee/Board Member.
Jana Hashash: BMS – Ad Board.
Mohamed Eldesouki, MD1, Ahmed Ibrahim, MD2, Mohammed Y. Youssef, MD3, Mohammad Kloub, MD4, Mona Ahmed, 5, Francis A.. Farraye, MD, MSc, MACG6, Jana G. Hashash, MD, MSc, FACG6. P1076 - Severe Influenza-Related Outcomes in Patients With Inflammatory Bowel Disease on Immunosuppressive Therapy: A Propensity-Matched Cohort Analysis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Saint Michael's Medical Center, New York Medical College, Newark, NJ; 2Medical University of South Carolina, Charleston, SC; 3Hunt Regional Medical Center, Greenville, TX; 4New York Medical College - Saint Michael's Medical Center, Bloomfield, NJ; 5Mansoura University School of Medicine, East Newark, NJ; 6Mayo Clinic, Jacksonville, FL
Introduction: Patients with inflammatory bowel disease (IBD) often require immunosuppressive therapy to induce and maintain remission. However, the impact of recent immunosuppression on the severity of influenza-related complications needs to be quantitated. We aimed to evaluate whether recent immunosuppressive therapy increases the risk of these complications.
Methods: We used the TriNetX Analytics Network, to examine adults (≥18 years) with a diagnosis of Crohn’s disease (K50.x) or ulcerative colitis (K51.x) during the 2022–2023 influenza season. Patients with recent immunosuppressive therapy (within 6 months) were compared to those without, using 1:1 propensity score matching (PSM) to balance baseline characteristics. Outcomes assessed included influenza infection and related complications during the influenza season.
Results: A total of 93,793 patients were identified, including 39,741 in the immunosuppressive group and 44,795 in the non-immunosuppressive IBD group. After PSM, each group had 39,741 patients (mean [SD] age, 50 [18] years; 54% women). The incidence of influenza diagnosis was significantly higher in the immunosuppressive group (7.1% vs 4.5%; HR, 1.51; 95% CI, 1.43–1.60; p< 0.001). Hospitalization rates were also increased (6.8% vs 4.5%; HR, 1.48; 95% CI, 1.38–1.58; p< 0.001), as were influenza-related complications (4.0% vs 2.5%; HR, 1.51; 95% CI, 1.39–1.63; p< 0.001). ICU admissions (2.4% vs 1.6%; HR, 1.38; 95% CI, 1.25–1.52; p< 0.001) and mechanical ventilation (0.7% vs 0.5%; HR, 1.32; 95% CI, 1.10–1.59; p=0.003) were both more frequent in the immunosuppressive group. Use of antiviral medications was higher among those in the immunosuppressive cohort (0.8% vs 0.3%; HR, 2.24; 95% CI, 1.82–2.76; p< 0.001), and overall mortality was modestly increased (1.1% vs 0.9%; HR, 1.17; 95% CI, 1.02–1.35; p=0.001). Subgroup analysis revealed the highest risks among patients on corticosteroids (HR 1.62, 95% CI 1.53–1.72), followed by JAK inhibitors (HR 1.53, 95% CI 1.43–1.63), and anti-IL-23 agents (HR 1.53, 95% CI 1.43–1.63). Anti-IL-12/23 and anti-TNF agents were also associated with elevated risks (Figure).
Discussion: Patients with IBD requiring immunosuppressive therapy had a significantly higher risk of influenza infection and severe complications compared to non-immunosuppressed counterparts. These findings highlight the importance of annual influenza vaccination and timely antiviral treatment in patients with IBD, particularly those on immunosuppressive therapy.

Figure: Table: Clinical features and outcomes
Abbreviations: IBD: inflammatory bowel disease; ICD: intensive care unit; SD: standard deviation; HR: hazard ratio.
*This included pneumonia, sepsis, acute bronchitis, myocarditis, and encephalopathy.

Figure: Figure. Forest plot depicting the risk of influenza infection in different immunosuppressive medications.
Disclosures:
Mohamed Eldesouki indicated no relevant financial relationships.
Ahmed Ibrahim indicated no relevant financial relationships.
Mohammed Y. Youssef indicated no relevant financial relationships.
Mohammad Kloub indicated no relevant financial relationships.
Mona Ahmed indicated no relevant financial relationships.
Francis Farraye: Astellas – Advisory Committee/Board Member. Avalo – Advisory Committee/Board Member. Bausch – Advisory Committee/Board Member. BMS – Advisory Committee/Board Member. Braintree Labs – Advisory Committee/Board Member. Fresenius Kabi – Advisory Committee/Board Member. GI Reviewers – Independent Contractor. IBD Educational Group – Independent Contractor. Iterative Health – Advisory Committee/Board Member, Stock Options. Janssen – Advisory Committee/Board Member. Lilly – DSMB. Pfizer – Advisory Committee/Board Member. Pharmacosmos – Advisory Committee/Board Member. Sandoz – Advisory Committee/Board Member. Viatris – Advisory Committee/Board Member.
Jana Hashash: BMS – Ad Board.
Mohamed Eldesouki, MD1, Ahmed Ibrahim, MD2, Mohammed Y. Youssef, MD3, Mohammad Kloub, MD4, Mona Ahmed, 5, Francis A.. Farraye, MD, MSc, MACG6, Jana G. Hashash, MD, MSc, FACG6. P1076 - Severe Influenza-Related Outcomes in Patients With Inflammatory Bowel Disease on Immunosuppressive Therapy: A Propensity-Matched Cohort Analysis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
