Sunday Poster Session
Category: IBD
P1068 - Restarting Etrasimod After Treatment Interruption Is Not Associated With Cardiovascular Events: Analysis From the ELEVATE UC Clinical Program
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall

David T. Rubin, MD
University of Chicago Medicine Inflammatory Bowel Disease Center, Chicago, IL, USA
Chicago, IL
Presenting Author(s)
David T. Rubin, MD1, Andres J. Yarur, MD2, Michael V. Chiorean, MD3, Aline Charabaty, MD, FACG4, Joseph Wu, PhD5, Krisztina Lazin, MD6, Micheal Keating, PharmD7, Arcangelo M. Abbatemarco, MD7, Silvio Danese, MD, PhD8
1University of Chicago Medicine Inflammatory Bowel Disease Center, Chicago, IL, USA, Chicago, IL; 2Inflammatory Bowel Disease Center and Division of Gastroenterology and Hepatology, Cedars-Sinai Medical Center, Los Angeles, CA, USA, Los Angeles, CA; 3Inflammatory Bowel Disease Center, Swedish Medical Center, Seattle, WA, USA, Seattle, WA; 4Johns Hopkins University School of Medicine, Washington, DC; 5Pfizer Inc, Cambridge, MA, USA, Cambridge, MA; 6Pfizer AG, Zürich, Switzerland, Zürich, Zurich, Switzerland; 7Pfizer Inc, New York, NY, USA, New York, NY; 8Gastroenterology and Endoscopy, IRCCS Ospedale San Raffaele and University Vita-Salute San Raffaele, Milan, Lombardia, Italy
Introduction: Etrasimod is an oral, once-daily (QD), selective sphingosine 1-phosphate (S1P)1,4,5 receptor modulator for the treatment of moderately to severe active ulcerative colitis (UC). First-dose heart rate lowering effects can occur with other S1P receptors, typically on Day 1.1 Cardiovascular (CV)-related treatment-emergent adverse events (TEAEs) in the ELEVATE UC clinical program were infrequent and mostly non-serious.2
Methods: We evaluated the CV safety of etrasimod 2 mg QD in patients (pts) in the ELEVATE UC clinical program who reinitiated etrasimod after a dose interruption > 7 consecutive days. The 7-day threshold was per protocol and based on the ~ 30-hour etrasimod half-life. TEAEs were recorded from Day 1 (first dose) and up to 21 days post-dose reinitiation. Per protocol, CV vital signs (heart rate and systolic and diastolic blood pressure [SBP; DBP]) were monitored at pre-dose and for ≥ 4 hours post-dose on Day 1 and at reinitiation after dose interruption.
Results: In this analysis, 25/747 (3.3%) pts experienced a dose interruption; 17 completed ELEVATE UC 52 or ELEVATE UC 12 or were ongoing in the open-label extension at the data cut (January 31, 2022). Mean time to first dose interruption was 205.5 days (standard deviation: 165.6); mean duration of interruption was 22.4 (10.9) days. Of TEAEs that led to dose interruption (22/25), decreased lymphocyte count (n = 8/25) was the most common; no CV events led to dose interruptions. No TEAEs were within 7 days of dose reinitiation; three (upper respiratory tract infection, lipomatosis and migraine) were reported within 21 days. No CV events were reported. Mean changes in heart rate and SBP and DBP were consistent at 4 hours post-dose between Day 1 and day of dose reinitiation (Table).
Discussion: Reinitiation of etrasimod after a treatment interruption > 7 consecutive days was not associated with bradycardia or AV block; no CV-related events were reported on the day of reinitiation or within 21 days. CV-related TEAEs were not associated with any dose interruptions. This suggests etrasimod can safely be reinitiated after interruption in pts with moderately to severely active UC once the reason for interruption is resolved.
References
1. Sandborn WJ et al. N Engl J Med 2021; 385: 1280–1291.
2. Vermeire S et al. BMJ Open Gastroenterol 2025; 12: e001516
Pfizer’s generative artificial intelligence tool MAIA was used to assist production of the abstract first draft. Authors reviewed/edited and take responsibility for the content.
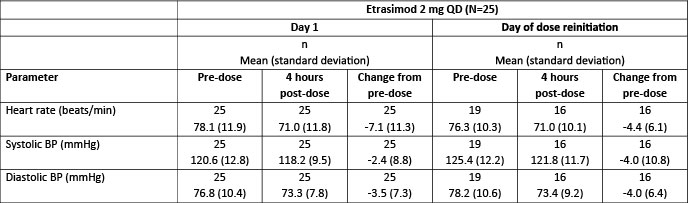
Figure: Table. Values and change from pre-dose (baseline) values in vital signs on Day 1 and on day of dose reinitiation in pts treated with etrasimod 2 mg QD with dose interruption > 7 consecutive days in the ELEVATE UC clinical program.
The ELEVATE UC clinical program includes ELEVATE UC 52 (NCT03945188), ELEVATE UC 12 (NCT03996369) and ELEVATE UC OLE (NCT03950232) (data cut January 31, 2022). Analyses were performed using the Safety Analysis Set. For pts with multiple dose interruptions, only the first occurrence with > 7 consecutive days is considered. Day 1 is the day of first dose of etrasimod 2 mg QD. Day of dose reinitiation is the day of etrasimod 2 mg dose reinitiation after the first etrasimod 2 mg dose interruption of > 7 consecutive days, with respect to Day 1.
BP, blood pressure; n, number of pts from which the parameter was measured; N, number of pts who took etrasimod 2 mg QD and had dose interruption > 7 consecutive days; OLE, open-label extension; pts, patients; QD, once daily; UC, ulcerative colitis.
Disclosures:
David Rubin: AbbVie – Advisory Committee/Board Member, Consultant, Speaker fees. Abivax SA – Consultant. Altrubio – Advisory Committee/Board Member, Consultant, Speaker feees, Stock Options. Avalo – Advisory Committee/Board Member, Consultant, Speaker fees. Bausch Health – Consultant. Bristol Myers Squibb – Advisory Committee/Board Member, Consultant, Speaker fees. Buhlmann Diagnostics – Advisory Committee/Board Member, Consultant, Speaker fees. Celltrion – Consultant. ClostraBio – Consultant. Connect BioPharma – Consultant. Cornerstones Health, Inc – Board of Directors membership. Douglas Pharmaceuticals – Consultant. Eli Lilly & Co. – Consultant. Foresee, Genentech (Roche) Inc. – Consultant. Image Analysis Group – Consultant. InDex Pharmaceutical – Consultant. Intouch Group – Advisory Committee/Board Member, Consultant, Speaker fees. Iterative Health – Advisory Committee/Board Member, Consultant, Speaker fees. Iterative Health – Stock Options. Janssen Pharmaceuticals – Consultant. Lilly – Advisory Committee/Board Member, Consultant, Speaker fees. Odyssey Therapeutics – Consultant. Pfizer – Advisory Committee/Board Member, Consultant, Speaker fees. Sanofi – Consultant. Takeda – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speaker fees. Throne – Consultant. Vedanta – Consultant.
Andres Yarur: AbbVie – Consultant, Speakers Bureau. Arena – Consultant. Bristol Myers Squibb – Consultant, Speakers Bureau. Celltrion – Consultant. Pfizer Inc – Consultant. Takeda – Consultant.
Michael Chiorean: AbbVie – Speakers Bureau. BMS – Consultant. Celltrion – Advisor or Review Panel Member, Speakers Bureau. Gilead – Advisor or Review Panel Member, Grant/Research Support. Johnson & Johnson – Advisor or Review Panel Member, Speakers Bureau. Lilly – Advisor or Review Panel Member, Consultant, Speakers Bureau. Merck – Consultant. Pfizer – Advisor or Review Panel Member, Consultant, Speakers Bureau. Takeda – Speakers Bureau.
Aline Charabaty: AbbVie – Advisory Committee/Board Member, Consultant. Boehringer Ingelheim – Advisory Committee/Board Member, Consultant. Celltrion – Grant/Research Support. Eli Lilly – Advisory Committee/Board Member, Consultant. guardant health – Consultant. Janssen – Advisory Committee/Board Member, Consultant. Pfizer Inc – Advisory Committee/Board Member, Consultant. sanofi – Advisor or Review Panel Member, Consultant. scrubs & heels foundation – co-founder. Takeda – Advisory Committee/Board Member, Consultant.
Joseph Wu: Pfizer Inc – Employee, Stock Options.
Krisztina Lazin: Pfizer AG – Employee. Pfizer Inc – Stock Options.
Micheal Keating: Pfizer Inc – Employee, Stock Options.
Arcangelo Abbatemarco: Pfizer Inc – Employee, Stock Options.
Silvio Danese: AbbVie – Consultant, Lecture fees. Alimentiv – Consultant. Allergan – Consultant. Amgen – Consultant, Lecture fees. AstraZeneca – Consultant. Athos Therapeutics – Consultant. Biogen – Consultant. Boehringer Ingelheim – Consultant. Celgene – Consultant. Celltrion – Consultant. Eli Lilly – Consultant. Enthera – Consultant. F. Hoffmann-La Roche Ltd – Consultant. Ferring Pharmaceuticals Inc. – Consultant, Lecture fees. Gilead – Consultant, Lecture fees. Hospira – Consultant. Inotrem – Consultant. Johnson & Johnson – Consultant, Lecture fees. MSD – Consultant. Mundipharma – Consultant. Mylan – Consultant, Lecture fees. Pfizer – Consultant, Lecture fees. Sandoz – Consultant. Sublimity Therapeutics – Consultant. Takeda – Consultant, Lecture fees. TiGenix – Consultant. UCB Inc. – Consultant. Vifor (International) Ltd. – Consultant.
David T. Rubin, MD1, Andres J. Yarur, MD2, Michael V. Chiorean, MD3, Aline Charabaty, MD, FACG4, Joseph Wu, PhD5, Krisztina Lazin, MD6, Micheal Keating, PharmD7, Arcangelo M. Abbatemarco, MD7, Silvio Danese, MD, PhD8. P1068 - Restarting Etrasimod After Treatment Interruption Is Not Associated With Cardiovascular Events: Analysis From the ELEVATE UC Clinical Program, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1University of Chicago Medicine Inflammatory Bowel Disease Center, Chicago, IL, USA, Chicago, IL; 2Inflammatory Bowel Disease Center and Division of Gastroenterology and Hepatology, Cedars-Sinai Medical Center, Los Angeles, CA, USA, Los Angeles, CA; 3Inflammatory Bowel Disease Center, Swedish Medical Center, Seattle, WA, USA, Seattle, WA; 4Johns Hopkins University School of Medicine, Washington, DC; 5Pfizer Inc, Cambridge, MA, USA, Cambridge, MA; 6Pfizer AG, Zürich, Switzerland, Zürich, Zurich, Switzerland; 7Pfizer Inc, New York, NY, USA, New York, NY; 8Gastroenterology and Endoscopy, IRCCS Ospedale San Raffaele and University Vita-Salute San Raffaele, Milan, Lombardia, Italy
Introduction: Etrasimod is an oral, once-daily (QD), selective sphingosine 1-phosphate (S1P)1,4,5 receptor modulator for the treatment of moderately to severe active ulcerative colitis (UC). First-dose heart rate lowering effects can occur with other S1P receptors, typically on Day 1.1 Cardiovascular (CV)-related treatment-emergent adverse events (TEAEs) in the ELEVATE UC clinical program were infrequent and mostly non-serious.2
Methods: We evaluated the CV safety of etrasimod 2 mg QD in patients (pts) in the ELEVATE UC clinical program who reinitiated etrasimod after a dose interruption > 7 consecutive days. The 7-day threshold was per protocol and based on the ~ 30-hour etrasimod half-life. TEAEs were recorded from Day 1 (first dose) and up to 21 days post-dose reinitiation. Per protocol, CV vital signs (heart rate and systolic and diastolic blood pressure [SBP; DBP]) were monitored at pre-dose and for ≥ 4 hours post-dose on Day 1 and at reinitiation after dose interruption.
Results: In this analysis, 25/747 (3.3%) pts experienced a dose interruption; 17 completed ELEVATE UC 52 or ELEVATE UC 12 or were ongoing in the open-label extension at the data cut (January 31, 2022). Mean time to first dose interruption was 205.5 days (standard deviation: 165.6); mean duration of interruption was 22.4 (10.9) days. Of TEAEs that led to dose interruption (22/25), decreased lymphocyte count (n = 8/25) was the most common; no CV events led to dose interruptions. No TEAEs were within 7 days of dose reinitiation; three (upper respiratory tract infection, lipomatosis and migraine) were reported within 21 days. No CV events were reported. Mean changes in heart rate and SBP and DBP were consistent at 4 hours post-dose between Day 1 and day of dose reinitiation (Table).
Discussion: Reinitiation of etrasimod after a treatment interruption > 7 consecutive days was not associated with bradycardia or AV block; no CV-related events were reported on the day of reinitiation or within 21 days. CV-related TEAEs were not associated with any dose interruptions. This suggests etrasimod can safely be reinitiated after interruption in pts with moderately to severely active UC once the reason for interruption is resolved.
References
1. Sandborn WJ et al. N Engl J Med 2021; 385: 1280–1291.
2. Vermeire S et al. BMJ Open Gastroenterol 2025; 12: e001516
Pfizer’s generative artificial intelligence tool MAIA was used to assist production of the abstract first draft. Authors reviewed/edited and take responsibility for the content.

Figure: Table. Values and change from pre-dose (baseline) values in vital signs on Day 1 and on day of dose reinitiation in pts treated with etrasimod 2 mg QD with dose interruption > 7 consecutive days in the ELEVATE UC clinical program.
The ELEVATE UC clinical program includes ELEVATE UC 52 (NCT03945188), ELEVATE UC 12 (NCT03996369) and ELEVATE UC OLE (NCT03950232) (data cut January 31, 2022). Analyses were performed using the Safety Analysis Set. For pts with multiple dose interruptions, only the first occurrence with > 7 consecutive days is considered. Day 1 is the day of first dose of etrasimod 2 mg QD. Day of dose reinitiation is the day of etrasimod 2 mg dose reinitiation after the first etrasimod 2 mg dose interruption of > 7 consecutive days, with respect to Day 1.
BP, blood pressure; n, number of pts from which the parameter was measured; N, number of pts who took etrasimod 2 mg QD and had dose interruption > 7 consecutive days; OLE, open-label extension; pts, patients; QD, once daily; UC, ulcerative colitis.
Disclosures:
David Rubin: AbbVie – Advisory Committee/Board Member, Consultant, Speaker fees. Abivax SA – Consultant. Altrubio – Advisory Committee/Board Member, Consultant, Speaker feees, Stock Options. Avalo – Advisory Committee/Board Member, Consultant, Speaker fees. Bausch Health – Consultant. Bristol Myers Squibb – Advisory Committee/Board Member, Consultant, Speaker fees. Buhlmann Diagnostics – Advisory Committee/Board Member, Consultant, Speaker fees. Celltrion – Consultant. ClostraBio – Consultant. Connect BioPharma – Consultant. Cornerstones Health, Inc – Board of Directors membership. Douglas Pharmaceuticals – Consultant. Eli Lilly & Co. – Consultant. Foresee, Genentech (Roche) Inc. – Consultant. Image Analysis Group – Consultant. InDex Pharmaceutical – Consultant. Intouch Group – Advisory Committee/Board Member, Consultant, Speaker fees. Iterative Health – Advisory Committee/Board Member, Consultant, Speaker fees. Iterative Health – Stock Options. Janssen Pharmaceuticals – Consultant. Lilly – Advisory Committee/Board Member, Consultant, Speaker fees. Odyssey Therapeutics – Consultant. Pfizer – Advisory Committee/Board Member, Consultant, Speaker fees. Sanofi – Consultant. Takeda – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speaker fees. Throne – Consultant. Vedanta – Consultant.
Andres Yarur: AbbVie – Consultant, Speakers Bureau. Arena – Consultant. Bristol Myers Squibb – Consultant, Speakers Bureau. Celltrion – Consultant. Pfizer Inc – Consultant. Takeda – Consultant.
Michael Chiorean: AbbVie – Speakers Bureau. BMS – Consultant. Celltrion – Advisor or Review Panel Member, Speakers Bureau. Gilead – Advisor or Review Panel Member, Grant/Research Support. Johnson & Johnson – Advisor or Review Panel Member, Speakers Bureau. Lilly – Advisor or Review Panel Member, Consultant, Speakers Bureau. Merck – Consultant. Pfizer – Advisor or Review Panel Member, Consultant, Speakers Bureau. Takeda – Speakers Bureau.
Aline Charabaty: AbbVie – Advisory Committee/Board Member, Consultant. Boehringer Ingelheim – Advisory Committee/Board Member, Consultant. Celltrion – Grant/Research Support. Eli Lilly – Advisory Committee/Board Member, Consultant. guardant health – Consultant. Janssen – Advisory Committee/Board Member, Consultant. Pfizer Inc – Advisory Committee/Board Member, Consultant. sanofi – Advisor or Review Panel Member, Consultant. scrubs & heels foundation – co-founder. Takeda – Advisory Committee/Board Member, Consultant.
Joseph Wu: Pfizer Inc – Employee, Stock Options.
Krisztina Lazin: Pfizer AG – Employee. Pfizer Inc – Stock Options.
Micheal Keating: Pfizer Inc – Employee, Stock Options.
Arcangelo Abbatemarco: Pfizer Inc – Employee, Stock Options.
Silvio Danese: AbbVie – Consultant, Lecture fees. Alimentiv – Consultant. Allergan – Consultant. Amgen – Consultant, Lecture fees. AstraZeneca – Consultant. Athos Therapeutics – Consultant. Biogen – Consultant. Boehringer Ingelheim – Consultant. Celgene – Consultant. Celltrion – Consultant. Eli Lilly – Consultant. Enthera – Consultant. F. Hoffmann-La Roche Ltd – Consultant. Ferring Pharmaceuticals Inc. – Consultant, Lecture fees. Gilead – Consultant, Lecture fees. Hospira – Consultant. Inotrem – Consultant. Johnson & Johnson – Consultant, Lecture fees. MSD – Consultant. Mundipharma – Consultant. Mylan – Consultant, Lecture fees. Pfizer – Consultant, Lecture fees. Sandoz – Consultant. Sublimity Therapeutics – Consultant. Takeda – Consultant, Lecture fees. TiGenix – Consultant. UCB Inc. – Consultant. Vifor (International) Ltd. – Consultant.
David T. Rubin, MD1, Andres J. Yarur, MD2, Michael V. Chiorean, MD3, Aline Charabaty, MD, FACG4, Joseph Wu, PhD5, Krisztina Lazin, MD6, Micheal Keating, PharmD7, Arcangelo M. Abbatemarco, MD7, Silvio Danese, MD, PhD8. P1068 - Restarting Etrasimod After Treatment Interruption Is Not Associated With Cardiovascular Events: Analysis From the ELEVATE UC Clinical Program, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
