Sunday Poster Session
Category: IBD
P1054 - Clinical Effectiveness and Safety of GLP1RA in Patients With IBD
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall
- HS
Hamzah Shariff, MD
Thomas Jefferson University Hospital
Philadelphia, PA
Presenting Author(s)
Hamzah Shariff, MD1, Vincent Dioguardi, MD1, Joy Zhao, MD1, Jasmine Lee, MD1, Caleb Song, BS2, Vaishnavi Nara, BS3, Breanne McDermott, BS3, Cindy Xin Fang, BS3, Raina Shivashankar, MD1, Patricia L. Kozuch, MD1, Cuckoo Choudhary, MD4, Priya Sehgal, MD1
1Thomas Jefferson University Hospital, Philadelphia, PA; 2Sidney Kimmel Medical College At Thomas Jefferson University, Philadelphia, PA; 3Sidney Kimmel Medical College at Thomas Jefferson University, Philadelphia, PA; 4Thomas Jefferson University Hospital (Philadelphia, PA), Philadelphia, PA
Introduction: Glucagon-like peptide-1 receptor agonists (GLP-1RA) have garnered attention for their anti-inflammatory effects in patients with obesity and diabetes. Their use in inflammatory bowel disease (IBD) is of growing interest. However, the long-term impact of GLP-1RA–induced weight loss in IBD, particularly regarding safety and nutritional status, remains understudied. This study aims to assess weight loss and safety outcomes over a 12-month period in patients with IBD initiated on GLP-1RA therapy.
Methods: A retrospective chart review was conducted at a quaternary care center between January 2018 to January 2025 to identify patients with Crohn’s disease (CD) or ulcerative colitis (UC) treated with any GLP-1RA for at least 3 months. Baseline weight (within 6 months pre-initiation of GLP-1RA) was compared to weights at 3-month intervals up to at least 12 months post-initiation. Paired t-tests were used to assess weight changes. Adverse events, including GI symptoms and complications near initiation or dose changes, were recorded.
Results: A total of 360 IBD (180 CD and 174 UC) patients on a GLP-1RA were included and semaglutide was the most commonly prescribed GLP-1RA (57%). The sample had a median age of 56 with 64% females and 78% non-Hispanic White. Baseline weights were available for all patients (mean 102.88 kg (±24.62), with 165 having 12-month follow-up data. Statistically significant weight loss was observed at each 3-month interval compared to baseline prior to GLP-1RA initiation: 0–3 months (−3.12 kg), 3–6 months (−5.4 kg +/- 10.45), 6–9 months (−7.13 kg +/- 12.93), 9–12 months (−8.65 kg+/-13.88), and 12 months (−7.15 kg+/- 10.32), all p< 0.0001 (Table 1.). Adverse events were absent in 67% of patients with one episode of pancreatitis; reported GI symptoms included nausea/vomiting (13%), diarrhea (6%), and constipation (5.6%) (Table 2.).
Discussion: In this study, GLP-1RA use in patients with IBD was associated with significant and sustained weight loss over 12 months, reaching a peak reduction of 8.4% at 9–12 months. Notably, this occurred without clinical concern for malnutrition or sarcopenia. The treatment was well tolerated, with predominantly mild GI side effects. These findings suggest GLP-1RAs may offer a favorable safety profile while supporting potential long-term anti-inflammatory benefits in IBD through weight reduction. Further prospective studies may validate these outcomes by trending inflammatory markers and endoscopic changes.
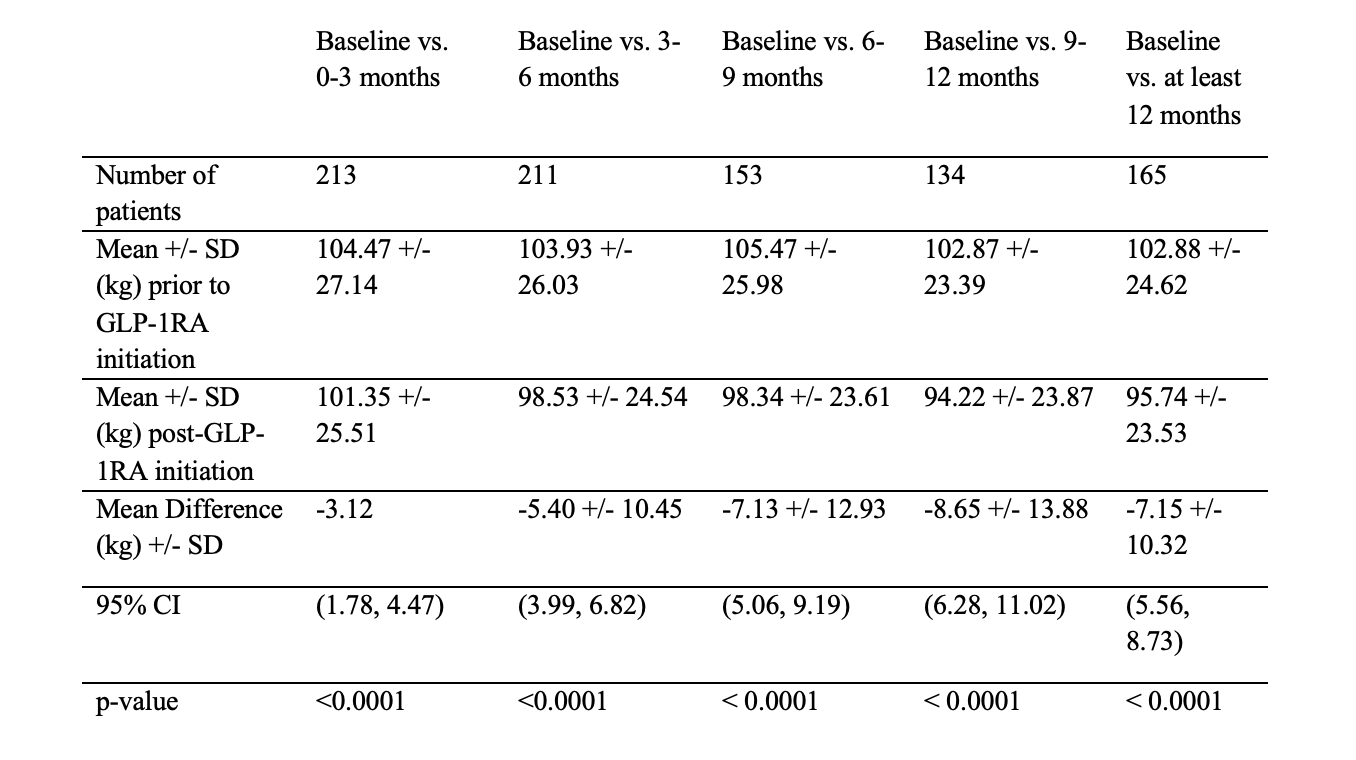
Figure: Table 1. Comparison of weight between pre- and post- GLP-1RA initiation within and at least 12 months
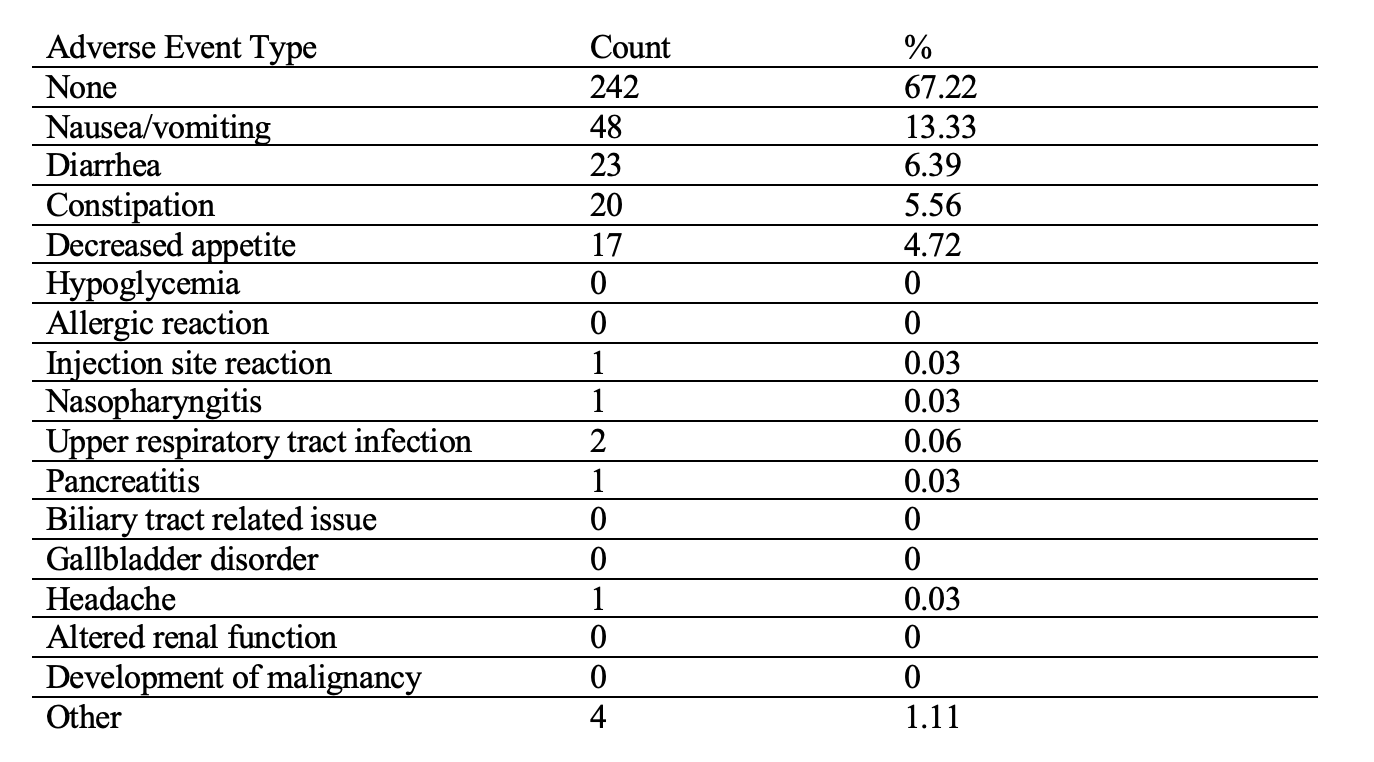
Figure: Table 2. Frequency of adverse events during treatment with GLP-1RA
Disclosures:
Hamzah Shariff indicated no relevant financial relationships.
Vincent Dioguardi indicated no relevant financial relationships.
Joy Zhao indicated no relevant financial relationships.
Jasmine Lee indicated no relevant financial relationships.
Caleb Song indicated no relevant financial relationships.
Vaishnavi Nara indicated no relevant financial relationships.
Breanne McDermott indicated no relevant financial relationships.
Cindy Xin Fang indicated no relevant financial relationships.
Raina Shivashankar: Abbvie – Speakers Bureau. BMS – Speakers Bureau. Janssen – Grant/Research Support. Pfizer – Consultant.
Patricia Kozuch indicated no relevant financial relationships.
Cuckoo Choudhary indicated no relevant financial relationships.
Priya Sehgal indicated no relevant financial relationships.
Hamzah Shariff, MD1, Vincent Dioguardi, MD1, Joy Zhao, MD1, Jasmine Lee, MD1, Caleb Song, BS2, Vaishnavi Nara, BS3, Breanne McDermott, BS3, Cindy Xin Fang, BS3, Raina Shivashankar, MD1, Patricia L. Kozuch, MD1, Cuckoo Choudhary, MD4, Priya Sehgal, MD1. P1054 - Clinical Effectiveness and Safety of GLP1RA in Patients With IBD, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Thomas Jefferson University Hospital, Philadelphia, PA; 2Sidney Kimmel Medical College At Thomas Jefferson University, Philadelphia, PA; 3Sidney Kimmel Medical College at Thomas Jefferson University, Philadelphia, PA; 4Thomas Jefferson University Hospital (Philadelphia, PA), Philadelphia, PA
Introduction: Glucagon-like peptide-1 receptor agonists (GLP-1RA) have garnered attention for their anti-inflammatory effects in patients with obesity and diabetes. Their use in inflammatory bowel disease (IBD) is of growing interest. However, the long-term impact of GLP-1RA–induced weight loss in IBD, particularly regarding safety and nutritional status, remains understudied. This study aims to assess weight loss and safety outcomes over a 12-month period in patients with IBD initiated on GLP-1RA therapy.
Methods: A retrospective chart review was conducted at a quaternary care center between January 2018 to January 2025 to identify patients with Crohn’s disease (CD) or ulcerative colitis (UC) treated with any GLP-1RA for at least 3 months. Baseline weight (within 6 months pre-initiation of GLP-1RA) was compared to weights at 3-month intervals up to at least 12 months post-initiation. Paired t-tests were used to assess weight changes. Adverse events, including GI symptoms and complications near initiation or dose changes, were recorded.
Results: A total of 360 IBD (180 CD and 174 UC) patients on a GLP-1RA were included and semaglutide was the most commonly prescribed GLP-1RA (57%). The sample had a median age of 56 with 64% females and 78% non-Hispanic White. Baseline weights were available for all patients (mean 102.88 kg (±24.62), with 165 having 12-month follow-up data. Statistically significant weight loss was observed at each 3-month interval compared to baseline prior to GLP-1RA initiation: 0–3 months (−3.12 kg), 3–6 months (−5.4 kg +/- 10.45), 6–9 months (−7.13 kg +/- 12.93), 9–12 months (−8.65 kg+/-13.88), and 12 months (−7.15 kg+/- 10.32), all p< 0.0001 (Table 1.). Adverse events were absent in 67% of patients with one episode of pancreatitis; reported GI symptoms included nausea/vomiting (13%), diarrhea (6%), and constipation (5.6%) (Table 2.).
Discussion: In this study, GLP-1RA use in patients with IBD was associated with significant and sustained weight loss over 12 months, reaching a peak reduction of 8.4% at 9–12 months. Notably, this occurred without clinical concern for malnutrition or sarcopenia. The treatment was well tolerated, with predominantly mild GI side effects. These findings suggest GLP-1RAs may offer a favorable safety profile while supporting potential long-term anti-inflammatory benefits in IBD through weight reduction. Further prospective studies may validate these outcomes by trending inflammatory markers and endoscopic changes.

Figure: Table 1. Comparison of weight between pre- and post- GLP-1RA initiation within and at least 12 months

Figure: Table 2. Frequency of adverse events during treatment with GLP-1RA
Disclosures:
Hamzah Shariff indicated no relevant financial relationships.
Vincent Dioguardi indicated no relevant financial relationships.
Joy Zhao indicated no relevant financial relationships.
Jasmine Lee indicated no relevant financial relationships.
Caleb Song indicated no relevant financial relationships.
Vaishnavi Nara indicated no relevant financial relationships.
Breanne McDermott indicated no relevant financial relationships.
Cindy Xin Fang indicated no relevant financial relationships.
Raina Shivashankar: Abbvie – Speakers Bureau. BMS – Speakers Bureau. Janssen – Grant/Research Support. Pfizer – Consultant.
Patricia Kozuch indicated no relevant financial relationships.
Cuckoo Choudhary indicated no relevant financial relationships.
Priya Sehgal indicated no relevant financial relationships.
Hamzah Shariff, MD1, Vincent Dioguardi, MD1, Joy Zhao, MD1, Jasmine Lee, MD1, Caleb Song, BS2, Vaishnavi Nara, BS3, Breanne McDermott, BS3, Cindy Xin Fang, BS3, Raina Shivashankar, MD1, Patricia L. Kozuch, MD1, Cuckoo Choudhary, MD4, Priya Sehgal, MD1. P1054 - Clinical Effectiveness and Safety of GLP1RA in Patients With IBD, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
