Sunday Poster Session
Category: IBD
P1029 - Semaglutide and Tirzepatide Have the Most Clinical Benefit Compared to Most Weight Loss Medications in Inflammatory Bowel Disease
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall

Himaben Gohil, DO, MSc (she/her/hers)
University at Buffalo
Buffalo, NY
Presenting Author(s)
Himaben Gohil, DO, MSc1, Xavier Zonna, DO1, Lan Nguyen, DO1, Randy Cheung, MD2, Erin Ly, MD1
1University at Buffalo, Buffalo, NY; 2University of Buffalo, Buffalo, NY
Introduction: Preliminary evidence indicates that anti-diabetic agents, including glucagon-like peptide-1 receptor agonists (GLP-1RA) and tirzepatide, are safe for use in patients with inflammatory bowel disease (IBD) and may have clinical benefits. A proposed mechanism behind these benefits is the reduction in systemic inflammation associated with weight loss. Thus, our study aims to evaluate the impact of weight loss medications on the progression of IBD.
Methods: A retrospective cohort analysis was conducted using TriNetX, a global database of healthcare organizations. Subjects were adults over the age of 18 with either ulcerative colitis or Crohn’s disease. The study included 6 groups: those on tirzepatide, semaglutide, liraglutide, orlistat, phentermine-topiramate, and naltrexone-bupropion. The control group was not exposed to any of these weight loss medications. Patients on multiple weight loss drugs were excluded. Each group was propensity score-matched to the control based on demographics, social determinants of health, co-morbidities, and medications history. Primary outcomes studied included steroid use, hospitalization, death, and IBD-linked surgery. Results shown in Table A are presented as risk differences, calculated as the percent risk of the outcome in the control group minus that in the treatment group.
Results: Tirzepatide and semaglutide showed significant risk reduction in all outcomes. Tirzepatide had the highest risk reduction in steroid use, hospitalization, and mortality compared to all the other agents. Liraglutide had significantly reduced risks in all categories except mortality. Orlistat had no significant benefit in any category. Phentermine-topiramate and Naltrexone-bupropion had significantly reduced risks in all variables but IBD-linked surgery.
Discussion: GLP1RA and tirzepatide had significant positive effects on the progression of IBD. In comparison, non-diabetic weight loss agents were associated with significant but modest risk reductions in some outcomes. More specifically, their impact on IBD-specific outcomes such as steroid use and IBD-related surgery was either minimal or absent. Thus, indicating these benefits may reflect overall health improvements rather than IBD-specific effects. In contrast, our findings suggest that GLP-1RA and tirzepatide may exert gut-specific anti-inflammatory effects possibly independent of weight loss, highlighting a promising avenue for future research.
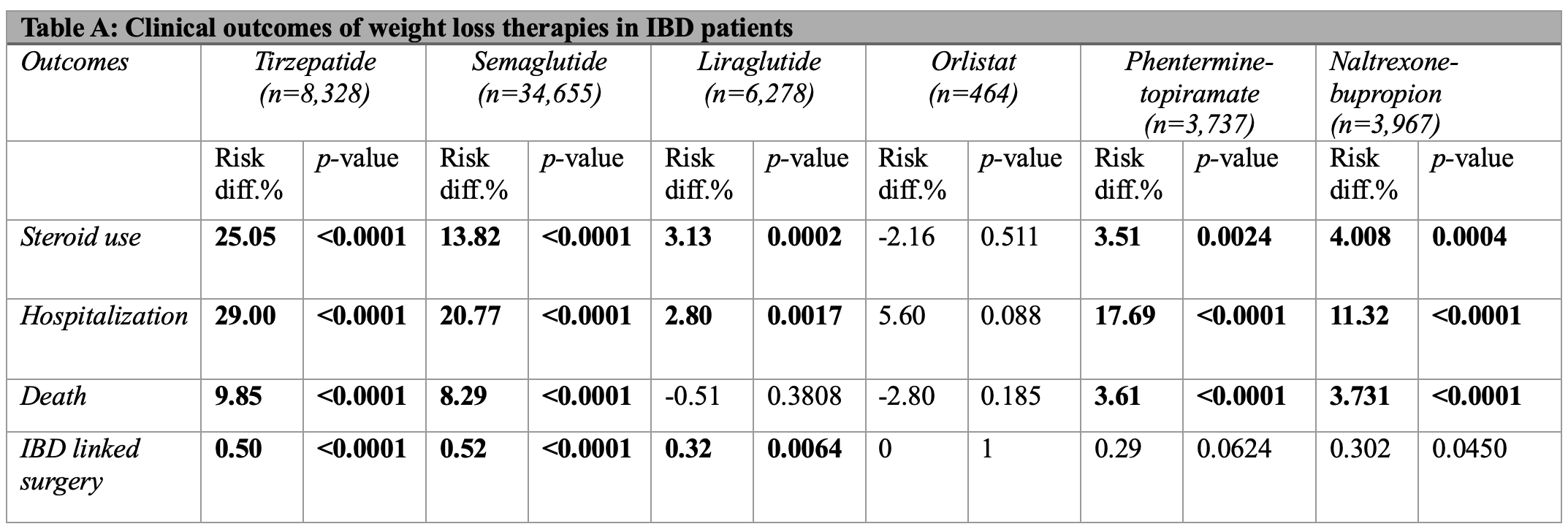
Figure: Table A: Clinical outcomes of weight loss therapies in IBD patients presented as risk differences. Risk difference is calculated as the percent risk of the outcome in the control group minus that in the treatment group. Bolded numbers are statistically significant (p < 0.05).
Disclosures:
Himaben Gohil indicated no relevant financial relationships.
Xavier Zonna indicated no relevant financial relationships.
Lan Nguyen indicated no relevant financial relationships.
Randy Cheung indicated no relevant financial relationships.
Erin Ly indicated no relevant financial relationships.
Himaben Gohil, DO, MSc1, Xavier Zonna, DO1, Lan Nguyen, DO1, Randy Cheung, MD2, Erin Ly, MD1. P1029 - Semaglutide and Tirzepatide Have the Most Clinical Benefit Compared to Most Weight Loss Medications in Inflammatory Bowel Disease, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1University at Buffalo, Buffalo, NY; 2University of Buffalo, Buffalo, NY
Introduction: Preliminary evidence indicates that anti-diabetic agents, including glucagon-like peptide-1 receptor agonists (GLP-1RA) and tirzepatide, are safe for use in patients with inflammatory bowel disease (IBD) and may have clinical benefits. A proposed mechanism behind these benefits is the reduction in systemic inflammation associated with weight loss. Thus, our study aims to evaluate the impact of weight loss medications on the progression of IBD.
Methods: A retrospective cohort analysis was conducted using TriNetX, a global database of healthcare organizations. Subjects were adults over the age of 18 with either ulcerative colitis or Crohn’s disease. The study included 6 groups: those on tirzepatide, semaglutide, liraglutide, orlistat, phentermine-topiramate, and naltrexone-bupropion. The control group was not exposed to any of these weight loss medications. Patients on multiple weight loss drugs were excluded. Each group was propensity score-matched to the control based on demographics, social determinants of health, co-morbidities, and medications history. Primary outcomes studied included steroid use, hospitalization, death, and IBD-linked surgery. Results shown in Table A are presented as risk differences, calculated as the percent risk of the outcome in the control group minus that in the treatment group.
Results: Tirzepatide and semaglutide showed significant risk reduction in all outcomes. Tirzepatide had the highest risk reduction in steroid use, hospitalization, and mortality compared to all the other agents. Liraglutide had significantly reduced risks in all categories except mortality. Orlistat had no significant benefit in any category. Phentermine-topiramate and Naltrexone-bupropion had significantly reduced risks in all variables but IBD-linked surgery.
Discussion: GLP1RA and tirzepatide had significant positive effects on the progression of IBD. In comparison, non-diabetic weight loss agents were associated with significant but modest risk reductions in some outcomes. More specifically, their impact on IBD-specific outcomes such as steroid use and IBD-related surgery was either minimal or absent. Thus, indicating these benefits may reflect overall health improvements rather than IBD-specific effects. In contrast, our findings suggest that GLP-1RA and tirzepatide may exert gut-specific anti-inflammatory effects possibly independent of weight loss, highlighting a promising avenue for future research.

Figure: Table A: Clinical outcomes of weight loss therapies in IBD patients presented as risk differences. Risk difference is calculated as the percent risk of the outcome in the control group minus that in the treatment group. Bolded numbers are statistically significant (p < 0.05).
Disclosures:
Himaben Gohil indicated no relevant financial relationships.
Xavier Zonna indicated no relevant financial relationships.
Lan Nguyen indicated no relevant financial relationships.
Randy Cheung indicated no relevant financial relationships.
Erin Ly indicated no relevant financial relationships.
Himaben Gohil, DO, MSc1, Xavier Zonna, DO1, Lan Nguyen, DO1, Randy Cheung, MD2, Erin Ly, MD1. P1029 - Semaglutide and Tirzepatide Have the Most Clinical Benefit Compared to Most Weight Loss Medications in Inflammatory Bowel Disease, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
