Sunday Poster Session
Category: GI Bleeding
P1012 - Capecitabine-Induced Recurrent Upper Gastrointestinal Hemorrhage
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall

Solomon Anighoro, MBBS
Guthrie Robert Packer Hospital
Sayre, PA
Presenting Author(s)
Solomon Anighoro, MBBS1, Rewanth Katamreddy, MD2, Matthew Lincoln, DO1, Chidera Onwuzo, MBBS3, Shravan ROBERT PACKER. Ponnekanti, 4
1Guthrie Robert Packer Hospital, Sayre, PA; 2Guthrie Robert Packer Hospital, Department of Gastroenterology, Sayre, PA; 3SUNY Upstate Medical University Hospital, Syracuse, NY; 4guthrie robert packer hospital, Sayre, PA
Introduction: Capecitabine is an oral prodrug of 5-fluorouracil (5-FU) widely used in the treatment of various solid tumors. While commonly associated side effects, including diarrhea, mucositis, and myelosuppression, serious gastrointestinal (GI) complications such as bleeding are rare and not well-characterized in the literature. GI bleeding from its use presents a diagnostic and management challenge, particularly in patients with underlying malignancy, polypharmacy, or comorbid gastrointestinal conditions. Here, we present a rare case of upper GI bleeding associated with capecitabine use in a patient undergoing chemotherapy, highlighting the importance of early recognition.
Case Description/
Methods: A 75-year-old male with recently diagnosed combined hepatocellular-cholangiocarcinoma (cHCC-ICC) presented with new-onset lower extremity weakness. He had undergone hepatectomy with lymph node dissection and was on neoadjuvant capecitabine. On day 2 of admission, he developed hematemesis and abdominal tenderness. Labs showed hemoglobin of 7.8 g/dL. EGD revealed pooled blood, clots, and multiple non-bleeding gastric ulcers with clean bases; the gastric mucosa was erythematous. Capecitabine-induced gastritis was suspected, and the drug was discontinued. He improved with supportive care and was discharged. Capecitabine was to be resumed at 50% dose, but he returned on the planned day with hematemesis, melena, hypotension, and hemoglobin of 6.8 g/dL. Repeat EGD showed multiple superficial ulcers and pigmented material in the cardia and fundus. He was again managed conservatively with resolution of symptoms.
Discussion: This case highlights a rare but significant GI complication following capecitabine initiation, occurring 12 days into treatment. Though the pathophysiology is unclear, it may involve mucosal toxicity or ischemia. Thymidine phosphorylase, which activates capecitabine, is highly expressed in the GI tract. The duration of onset of is not precisely defined in large cohort studies or guidelines, but available case reports and pharmacovigilance analyses indicate that such bleeding events typically occur within the first few cycles of therapy, often during the initial weeks of treatment, as seen in this patient. Early recognition is key to avoid misdiagnosis. Risk mitigation includes identifying high-risk patients (e.g., DPD deficiency, elderly, prior GI surgery), dose adjustments, and PPIs. A 4-week drug-free interval is advised post-bleed to allow for mucosal healing
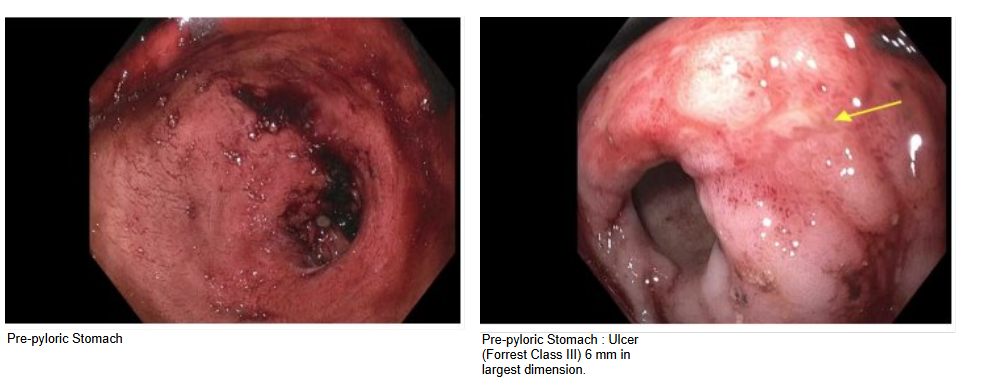
Figure: EGD findings showing pre-pyloric ulcer
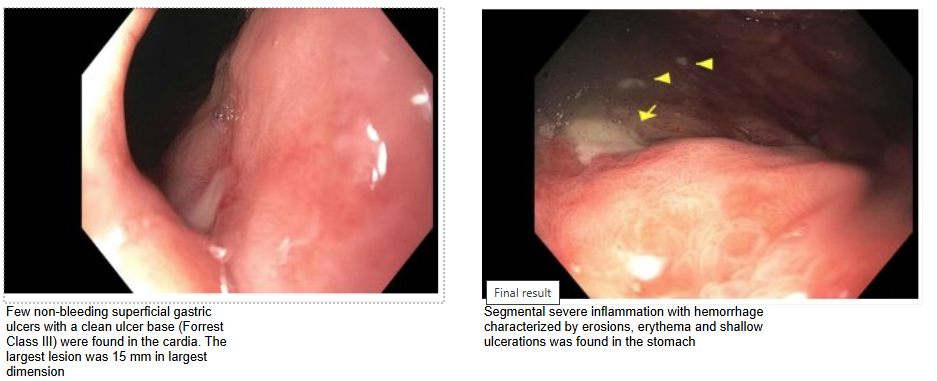
Figure: EGD findings showing ulcers in the gastric cardia and severe gastric mucosa inflammation
Disclosures:
Solomon Anighoro indicated no relevant financial relationships.
Rewanth Katamreddy indicated no relevant financial relationships.
Matthew Lincoln indicated no relevant financial relationships.
Chidera Onwuzo indicated no relevant financial relationships.
Shravan Ponnekanti indicated no relevant financial relationships.
Solomon Anighoro, MBBS1, Rewanth Katamreddy, MD2, Matthew Lincoln, DO1, Chidera Onwuzo, MBBS3, Shravan ROBERT PACKER. Ponnekanti, 4. P1012 - Capecitabine-Induced Recurrent Upper Gastrointestinal Hemorrhage, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Guthrie Robert Packer Hospital, Sayre, PA; 2Guthrie Robert Packer Hospital, Department of Gastroenterology, Sayre, PA; 3SUNY Upstate Medical University Hospital, Syracuse, NY; 4guthrie robert packer hospital, Sayre, PA
Introduction: Capecitabine is an oral prodrug of 5-fluorouracil (5-FU) widely used in the treatment of various solid tumors. While commonly associated side effects, including diarrhea, mucositis, and myelosuppression, serious gastrointestinal (GI) complications such as bleeding are rare and not well-characterized in the literature. GI bleeding from its use presents a diagnostic and management challenge, particularly in patients with underlying malignancy, polypharmacy, or comorbid gastrointestinal conditions. Here, we present a rare case of upper GI bleeding associated with capecitabine use in a patient undergoing chemotherapy, highlighting the importance of early recognition.
Case Description/
Methods: A 75-year-old male with recently diagnosed combined hepatocellular-cholangiocarcinoma (cHCC-ICC) presented with new-onset lower extremity weakness. He had undergone hepatectomy with lymph node dissection and was on neoadjuvant capecitabine. On day 2 of admission, he developed hematemesis and abdominal tenderness. Labs showed hemoglobin of 7.8 g/dL. EGD revealed pooled blood, clots, and multiple non-bleeding gastric ulcers with clean bases; the gastric mucosa was erythematous. Capecitabine-induced gastritis was suspected, and the drug was discontinued. He improved with supportive care and was discharged. Capecitabine was to be resumed at 50% dose, but he returned on the planned day with hematemesis, melena, hypotension, and hemoglobin of 6.8 g/dL. Repeat EGD showed multiple superficial ulcers and pigmented material in the cardia and fundus. He was again managed conservatively with resolution of symptoms.
Discussion: This case highlights a rare but significant GI complication following capecitabine initiation, occurring 12 days into treatment. Though the pathophysiology is unclear, it may involve mucosal toxicity or ischemia. Thymidine phosphorylase, which activates capecitabine, is highly expressed in the GI tract. The duration of onset of is not precisely defined in large cohort studies or guidelines, but available case reports and pharmacovigilance analyses indicate that such bleeding events typically occur within the first few cycles of therapy, often during the initial weeks of treatment, as seen in this patient. Early recognition is key to avoid misdiagnosis. Risk mitigation includes identifying high-risk patients (e.g., DPD deficiency, elderly, prior GI surgery), dose adjustments, and PPIs. A 4-week drug-free interval is advised post-bleed to allow for mucosal healing

Figure: EGD findings showing pre-pyloric ulcer

Figure: EGD findings showing ulcers in the gastric cardia and severe gastric mucosa inflammation
Disclosures:
Solomon Anighoro indicated no relevant financial relationships.
Rewanth Katamreddy indicated no relevant financial relationships.
Matthew Lincoln indicated no relevant financial relationships.
Chidera Onwuzo indicated no relevant financial relationships.
Shravan Ponnekanti indicated no relevant financial relationships.
Solomon Anighoro, MBBS1, Rewanth Katamreddy, MD2, Matthew Lincoln, DO1, Chidera Onwuzo, MBBS3, Shravan ROBERT PACKER. Ponnekanti, 4. P1012 - Capecitabine-Induced Recurrent Upper Gastrointestinal Hemorrhage, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
