Sunday Poster Session
Category: GI Bleeding
P0927 - Use of a Blood Detection Capsule to Guide Endoscopic Decision-Making in High-Risk Patients With Suspected GI Bleed
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall

Hadi Khaled Abou Zeid, MD
Mayo Clinic
Rochester, MN
Presenting Author(s)
Award: ACG Presidential Poster Award
Hadi Khaled. Abou Zeid, MD, David H. Bruining, MD, Navtej Buttar, MD, Louis Wong Kee Song, MD, Nayantara Coelho-Prabhu, MBBS, FACG, Cadman Leggett, MD, Mark Larson, MD, Andrew Storm, MD
Mayo Clinic, Rochester, MN
Introduction: Upper Endoscopy is generally a safe procedure, however specific patient populations, such as critically ill patients and those with severe cardiopulmonary comorbidities are at higher risk of complications. A novel ingestible capsule enables early detection of upper GI bleeding through a minimally invasive approach. This study aimed to evaluate the impact of this capsule on the management of patients who were high-risk for upper endoscopy.
Methods: We conducted a retrospective study to assess the clinical application of a novel blood detection capsule in patients with a high-risk for upper endoscopy complications. Patients with suspected GI bleeding and considered high-risk for upper endoscopy complications were included. Capsule results were used to inform diagnostic and management strategies. Patients were stratified by capsule result (positive vs negative), and clinical outcomes including Glasgow-Blatchford Bleeding Score, transfusion requirement, and hospital length of stay were compared using the Wilcoxon rank-sum test. The impact of the capsule on clinical decision-making was also evaluated.
Results: 20 patients were included (mean age 64.6 ± 16.2 years, 85% male). 13 (65%) were in a critical care setting and 7 (35%) were regarded as high-risk for sedation-related complications. Capsule results impacted clinical management in 14/20 (70%) cases. Upper endoscopy was deferred in 8 (40%) patients, prioritized in 5 (25%), and 1 patient (5%) was intubated prior to endoscopy for airway protection because they expected large volume of blood due to the immediate detection of blood by the capsule. Among patients in which upper endoscopy was deferred, none required subsequent endoscopy, experienced readmission for gastrointestinal bleeding, or died within 30 days. Patients with a positive capsule result had higher transfusion requirements (15.9 ± 31.9 vs. 2.8 ± 3.4 units, p = 0.32), higher Glasgow-Blatchford scores (15.1 ± 2.5 vs. 12.6 ± 4.2; p = 0.11), and longer hospital stays (29.9 ± 57.9 vs. 7.2 ± 3.0 days, p = 0.34).
Discussion: Use of the capsule guided clinical management helping to avoid or prioritize endoscopy in high-risk patients. While larger studies are needed, this device may enhance decision-making and resource allocation in the acute care of patients with suspected upper GI bleeding.
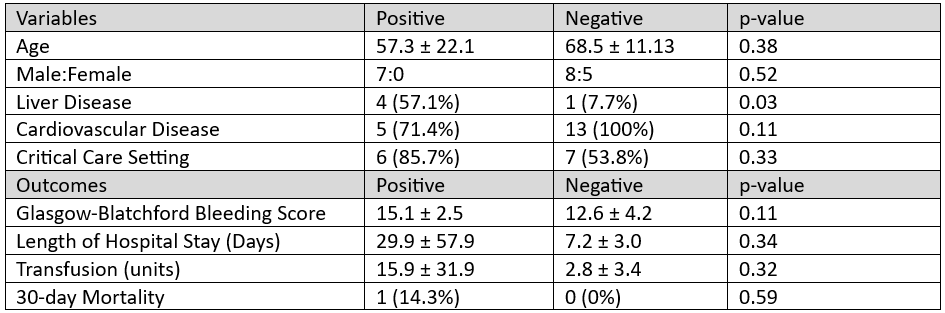
Figure: Table 1. Patient Characteristics and Outcomes
Disclosures:
Hadi Abou Zeid indicated no relevant financial relationships.
David Bruining: Johnson & Johnson – Consultant.
Navtej Buttar indicated no relevant financial relationships.
Louis Wong Kee Song: Noah Medical, Inc. – Consultant. Olympus Corp. – Consultant. Steris Inc. – Consultant.
Nayantara Coelho-Prabhu indicated no relevant financial relationships.
Cadman Leggett indicated no relevant financial relationships.
Mark Larson indicated no relevant financial relationships.
Andrew Storm: Ambu – Consultant. Apollo Endosurgery – Consultant, Grant/Research Support. Boston Scientific – Consultant, Grant/Research Support. Cook – Consultant. Endogenex – Grant/Research Support. Endo-Tagss – Grant/Research Support. Enterasense – Grant/Research Support. Envision Endoscopy – Grant/Research Support. Intuitive – Consultant. Medtronic – Consultant. MGI Medical – Grant/Research Support. Microtech – Consultant. Olympus – Consultant. OnePass – Grant/Research Support. SofTac – Grant/Research Support. Sotelix – Consultant. Steris – Consultant.
Hadi Khaled. Abou Zeid, MD, David H. Bruining, MD, Navtej Buttar, MD, Louis Wong Kee Song, MD, Nayantara Coelho-Prabhu, MBBS, FACG, Cadman Leggett, MD, Mark Larson, MD, Andrew Storm, MD. P0927 - Use of a Blood Detection Capsule to Guide Endoscopic Decision-Making in High-Risk Patients With Suspected GI Bleed, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
Hadi Khaled. Abou Zeid, MD, David H. Bruining, MD, Navtej Buttar, MD, Louis Wong Kee Song, MD, Nayantara Coelho-Prabhu, MBBS, FACG, Cadman Leggett, MD, Mark Larson, MD, Andrew Storm, MD
Mayo Clinic, Rochester, MN
Introduction: Upper Endoscopy is generally a safe procedure, however specific patient populations, such as critically ill patients and those with severe cardiopulmonary comorbidities are at higher risk of complications. A novel ingestible capsule enables early detection of upper GI bleeding through a minimally invasive approach. This study aimed to evaluate the impact of this capsule on the management of patients who were high-risk for upper endoscopy.
Methods: We conducted a retrospective study to assess the clinical application of a novel blood detection capsule in patients with a high-risk for upper endoscopy complications. Patients with suspected GI bleeding and considered high-risk for upper endoscopy complications were included. Capsule results were used to inform diagnostic and management strategies. Patients were stratified by capsule result (positive vs negative), and clinical outcomes including Glasgow-Blatchford Bleeding Score, transfusion requirement, and hospital length of stay were compared using the Wilcoxon rank-sum test. The impact of the capsule on clinical decision-making was also evaluated.
Results: 20 patients were included (mean age 64.6 ± 16.2 years, 85% male). 13 (65%) were in a critical care setting and 7 (35%) were regarded as high-risk for sedation-related complications. Capsule results impacted clinical management in 14/20 (70%) cases. Upper endoscopy was deferred in 8 (40%) patients, prioritized in 5 (25%), and 1 patient (5%) was intubated prior to endoscopy for airway protection because they expected large volume of blood due to the immediate detection of blood by the capsule. Among patients in which upper endoscopy was deferred, none required subsequent endoscopy, experienced readmission for gastrointestinal bleeding, or died within 30 days. Patients with a positive capsule result had higher transfusion requirements (15.9 ± 31.9 vs. 2.8 ± 3.4 units, p = 0.32), higher Glasgow-Blatchford scores (15.1 ± 2.5 vs. 12.6 ± 4.2; p = 0.11), and longer hospital stays (29.9 ± 57.9 vs. 7.2 ± 3.0 days, p = 0.34).
Discussion: Use of the capsule guided clinical management helping to avoid or prioritize endoscopy in high-risk patients. While larger studies are needed, this device may enhance decision-making and resource allocation in the acute care of patients with suspected upper GI bleeding.

Figure: Table 1. Patient Characteristics and Outcomes
Disclosures:
Hadi Abou Zeid indicated no relevant financial relationships.
David Bruining: Johnson & Johnson – Consultant.
Navtej Buttar indicated no relevant financial relationships.
Louis Wong Kee Song: Noah Medical, Inc. – Consultant. Olympus Corp. – Consultant. Steris Inc. – Consultant.
Nayantara Coelho-Prabhu indicated no relevant financial relationships.
Cadman Leggett indicated no relevant financial relationships.
Mark Larson indicated no relevant financial relationships.
Andrew Storm: Ambu – Consultant. Apollo Endosurgery – Consultant, Grant/Research Support. Boston Scientific – Consultant, Grant/Research Support. Cook – Consultant. Endogenex – Grant/Research Support. Endo-Tagss – Grant/Research Support. Enterasense – Grant/Research Support. Envision Endoscopy – Grant/Research Support. Intuitive – Consultant. Medtronic – Consultant. MGI Medical – Grant/Research Support. Microtech – Consultant. Olympus – Consultant. OnePass – Grant/Research Support. SofTac – Grant/Research Support. Sotelix – Consultant. Steris – Consultant.
Hadi Khaled. Abou Zeid, MD, David H. Bruining, MD, Navtej Buttar, MD, Louis Wong Kee Song, MD, Nayantara Coelho-Prabhu, MBBS, FACG, Cadman Leggett, MD, Mark Larson, MD, Andrew Storm, MD. P0927 - Use of a Blood Detection Capsule to Guide Endoscopic Decision-Making in High-Risk Patients With Suspected GI Bleed, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.

