Sunday Poster Session
Category: General Endoscopy
P0876 - Successful Management With Oral Budesonide to Treat Pan-Upper Gastrointestinal Toxicity Secondary to Immune Checkpoint Inhibitor Therapy
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall
- AS
Abdulmalik Saleem, MD
Henry Ford Hospital
Detroit, MI
Presenting Author(s)
Award: ACG Presidential Poster Award
Abdulmalik Saleem, MD1, Abdullah S. Shaikh, MD2, Sun Mi Lee, MD3, Hao Chi Zhang, MD2
1Henry Ford Hospital, Detroit, MI; 2University of Texas MD Anderson Cancer Center, Houston, TX; 3MD Anderson Cancer Center, Houston, TX
Introduction: Immune checkpoint inhibitors (ICIs) are well-known to cause upper and lower gastrointestinal (GI) toxicities, as an immune-related adverse event (irAE). Although cases of ICI-mediated gastritis have been described, simultaneous multifocal toxicity involving the esophagus, stomach, and small bowel is rarely reported. Oral budesonide is reported as a viable treatment option for both upper and lower GI toxicities. We present a case of a multifocal upper GI irAE, associated with pembrolizumab, that responded successfully to oral budesonide.
Case Description/
Methods: A 56-year-old woman with breast cancer, treated with doxorubicin/cyclophosphamide/pembrolizumab, presented with intractable nausea, vomiting, dysphagia to solids, and 15-pound weight loss, at 6 weeks after her last treatment cycle. Supportive measures with ondansetron and metoclopramide were ineffective. Upper endoscopy revealed normal-appearing esophagus but mild erythema in the stomach and duodenum. Histology from mucosal biopsies revealed active and chronic inflammation in the esophagus, stomach (without Helicobacter pylori detected), and duodenum. The diagnosis supported an ICI-mediated cause. Esomeprazole was not sufficient to control the symptoms. Oral budesonide was started at 9 mg/d (for 14 days); within 48 hours, the patient's upper GI symptoms completely resolved. Budesonide was tapered to 6 mg/d for 14 days, and then 3 mg/d for 14 days. Surveillance upper endoscopy one week after completing the budesonide course revealed minimal residual erythema in all three organs with near complete histologic remission. Further immunotherapy was deferred by oncology due to patient’s irAEs from ICI therapy.
Discussion: Recognition of upper GI irAEs related to ICI therapy is increasing. Symptoms refractory to standard antiemetic therapy should raise suspicion for upper GI toxicity. Our case highlights a diagnostic challenge: Endoscopic findings may non-specific, mild, or even absent, despite severe symptoms and active histologic inflammation. Histologic evaluation remains essential for diagnosing ICI-mediated GI mucosal injury. Early recognition with endoscopy referral will allow for timely diagnosis and treatment to prevent sequelae and allow patients to recover and resume cancer treatment. Systemic corticosteroids are not always necessary. Oral budesonide, with high topical activity and low systemic absorption, can be very effective. Further studies are needed regarding the effectiveness and role of budesonide in management of GI irAEs.
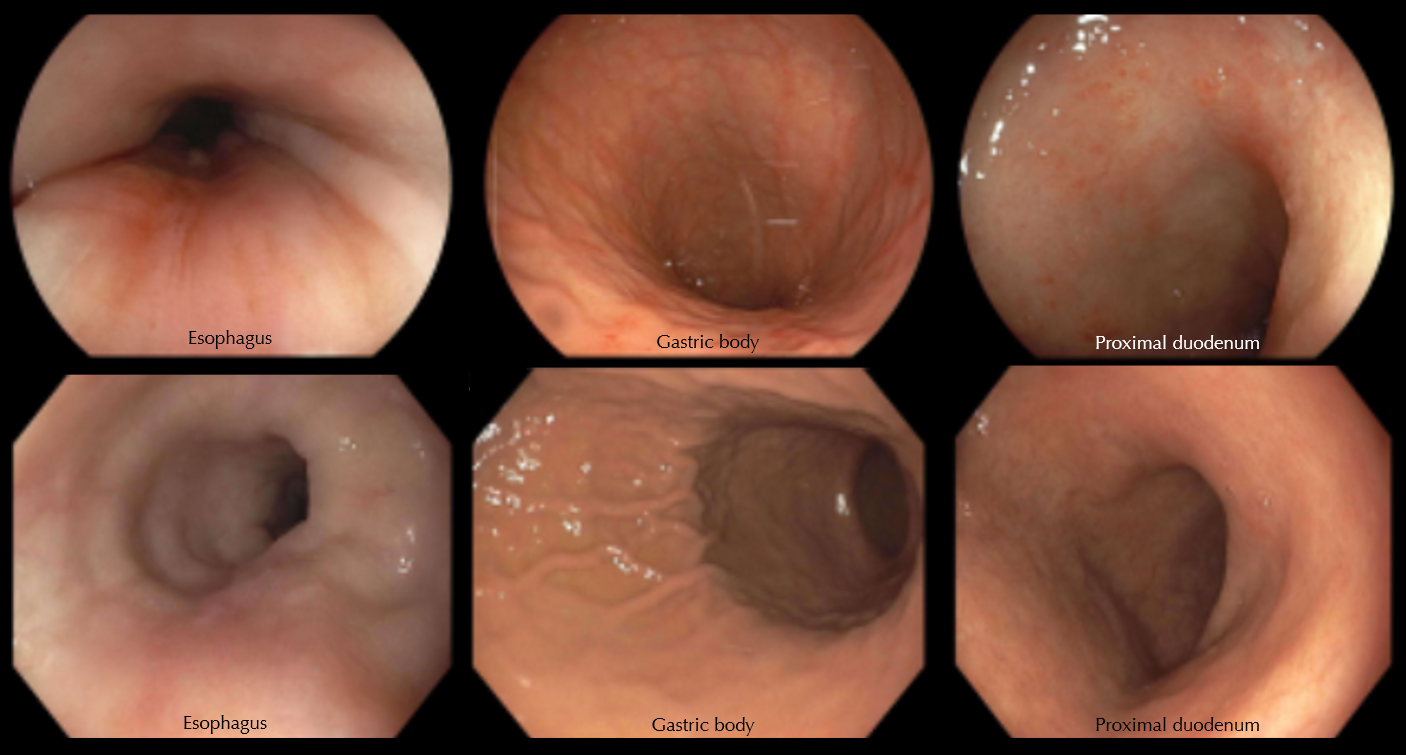
Figure: Figure 1.
Top row (left to right): Representative endoscopic views of the esophagus, stomach, and duodenum, as seen on the initial upper endoscopy. The esophagus appeared normal. There was mild erythema throughout the gastric body without frank ulcerations. There was mild erythema of the proximal duodenum.
Bottom row (left to right): Representative endoscopic views of the esophagus, stomach, and duodenum, as seen on the follow-up upper endoscopic examination after completion of budesonide treatment. The visual appearance of the mucosa in all three regions was normal.
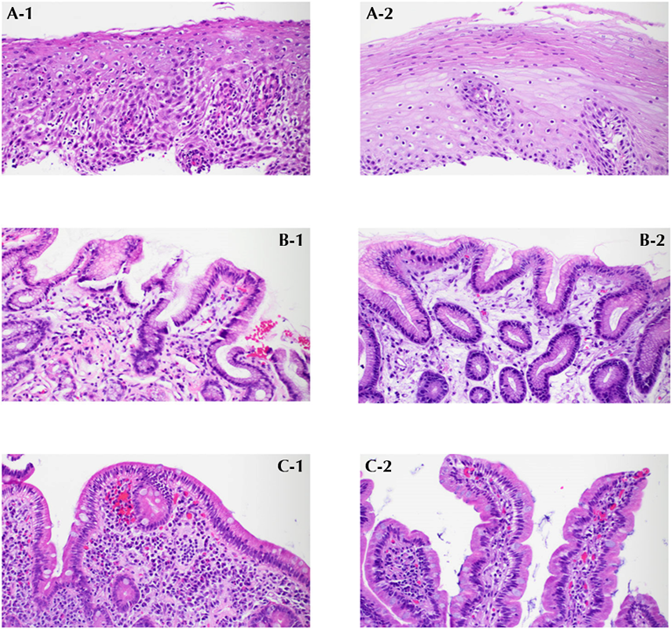
Figure: Figure 2.
A-1. After the application of an immune checkpoint inhibitor (ICI), the squamous epithelium of the esophagus reveals occasional apoptotic keratinocytes, intercellular edema, and dense lymphocytic infiltration, consistent with ICI-associated mucosal injury, with no clinical or pathologic evidence of infections.
A-2. After treatment with budesonide, the dense lymphocytic infiltrates, apoptotic keratinocytes, and edema in the squamous epithelium of the esophagus resolve.
B-1. After ICI treatment, the gastric mucosa demonstrates extensive mucosal edema, intramucosal hemorrhage and congestion, as well as lymphoplasmacytic infiltration in the lamina propria, consistent with ICI-associated mucosal injury.
B-2. After treatment with budesonide, the gastric mucosa shows only a few scattered plasma cells in the lamina propria.
C-1. After ICI treatment, the duodenal mucosa reveals focal villous blunting, increased inflammatory cells with plasma cells and eosinophils, along with congestion and edema, consistent with ICI-associated mucosal injury.
C-2. After treatment with budesonide, the duodenal mucosa exhibits normal villous architecture and a normal distribution of plasma cells and lymphocytes.
Disclosures:
Abdulmalik Saleem indicated no relevant financial relationships.
Abdullah Shaikh indicated no relevant financial relationships.
Sun Mi Lee indicated no relevant financial relationships.
Hao Chi Zhang indicated no relevant financial relationships.
Abdulmalik Saleem, MD1, Abdullah S. Shaikh, MD2, Sun Mi Lee, MD3, Hao Chi Zhang, MD2. P0876 - Successful Management With Oral Budesonide to Treat Pan-Upper Gastrointestinal Toxicity Secondary to Immune Checkpoint Inhibitor Therapy, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
Abdulmalik Saleem, MD1, Abdullah S. Shaikh, MD2, Sun Mi Lee, MD3, Hao Chi Zhang, MD2
1Henry Ford Hospital, Detroit, MI; 2University of Texas MD Anderson Cancer Center, Houston, TX; 3MD Anderson Cancer Center, Houston, TX
Introduction: Immune checkpoint inhibitors (ICIs) are well-known to cause upper and lower gastrointestinal (GI) toxicities, as an immune-related adverse event (irAE). Although cases of ICI-mediated gastritis have been described, simultaneous multifocal toxicity involving the esophagus, stomach, and small bowel is rarely reported. Oral budesonide is reported as a viable treatment option for both upper and lower GI toxicities. We present a case of a multifocal upper GI irAE, associated with pembrolizumab, that responded successfully to oral budesonide.
Case Description/
Methods: A 56-year-old woman with breast cancer, treated with doxorubicin/cyclophosphamide/pembrolizumab, presented with intractable nausea, vomiting, dysphagia to solids, and 15-pound weight loss, at 6 weeks after her last treatment cycle. Supportive measures with ondansetron and metoclopramide were ineffective. Upper endoscopy revealed normal-appearing esophagus but mild erythema in the stomach and duodenum. Histology from mucosal biopsies revealed active and chronic inflammation in the esophagus, stomach (without Helicobacter pylori detected), and duodenum. The diagnosis supported an ICI-mediated cause. Esomeprazole was not sufficient to control the symptoms. Oral budesonide was started at 9 mg/d (for 14 days); within 48 hours, the patient's upper GI symptoms completely resolved. Budesonide was tapered to 6 mg/d for 14 days, and then 3 mg/d for 14 days. Surveillance upper endoscopy one week after completing the budesonide course revealed minimal residual erythema in all three organs with near complete histologic remission. Further immunotherapy was deferred by oncology due to patient’s irAEs from ICI therapy.
Discussion: Recognition of upper GI irAEs related to ICI therapy is increasing. Symptoms refractory to standard antiemetic therapy should raise suspicion for upper GI toxicity. Our case highlights a diagnostic challenge: Endoscopic findings may non-specific, mild, or even absent, despite severe symptoms and active histologic inflammation. Histologic evaluation remains essential for diagnosing ICI-mediated GI mucosal injury. Early recognition with endoscopy referral will allow for timely diagnosis and treatment to prevent sequelae and allow patients to recover and resume cancer treatment. Systemic corticosteroids are not always necessary. Oral budesonide, with high topical activity and low systemic absorption, can be very effective. Further studies are needed regarding the effectiveness and role of budesonide in management of GI irAEs.

Figure: Figure 1.
Top row (left to right): Representative endoscopic views of the esophagus, stomach, and duodenum, as seen on the initial upper endoscopy. The esophagus appeared normal. There was mild erythema throughout the gastric body without frank ulcerations. There was mild erythema of the proximal duodenum.
Bottom row (left to right): Representative endoscopic views of the esophagus, stomach, and duodenum, as seen on the follow-up upper endoscopic examination after completion of budesonide treatment. The visual appearance of the mucosa in all three regions was normal.

Figure: Figure 2.
A-1. After the application of an immune checkpoint inhibitor (ICI), the squamous epithelium of the esophagus reveals occasional apoptotic keratinocytes, intercellular edema, and dense lymphocytic infiltration, consistent with ICI-associated mucosal injury, with no clinical or pathologic evidence of infections.
A-2. After treatment with budesonide, the dense lymphocytic infiltrates, apoptotic keratinocytes, and edema in the squamous epithelium of the esophagus resolve.
B-1. After ICI treatment, the gastric mucosa demonstrates extensive mucosal edema, intramucosal hemorrhage and congestion, as well as lymphoplasmacytic infiltration in the lamina propria, consistent with ICI-associated mucosal injury.
B-2. After treatment with budesonide, the gastric mucosa shows only a few scattered plasma cells in the lamina propria.
C-1. After ICI treatment, the duodenal mucosa reveals focal villous blunting, increased inflammatory cells with plasma cells and eosinophils, along with congestion and edema, consistent with ICI-associated mucosal injury.
C-2. After treatment with budesonide, the duodenal mucosa exhibits normal villous architecture and a normal distribution of plasma cells and lymphocytes.
Disclosures:
Abdulmalik Saleem indicated no relevant financial relationships.
Abdullah Shaikh indicated no relevant financial relationships.
Sun Mi Lee indicated no relevant financial relationships.
Hao Chi Zhang indicated no relevant financial relationships.
Abdulmalik Saleem, MD1, Abdullah S. Shaikh, MD2, Sun Mi Lee, MD3, Hao Chi Zhang, MD2. P0876 - Successful Management With Oral Budesonide to Treat Pan-Upper Gastrointestinal Toxicity Secondary to Immune Checkpoint Inhibitor Therapy, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.


