Sunday Poster Session
Category: Functional Bowel Disease
P0832 - GLP-1 Agonists and Dyspeptic Symptoms: A Case Series in the Ambulatory Setting
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall
- CC
Christopher Costa, MD (he/him/his)
University of Connecticut Health
Rochester, MN
Presenting Author(s)
Christopher Costa, MD1, Hira Khan, MD2, Lisa Weisinger, MD3
1University of Connecticut Health, Rochester, MN; 2University of Connecticut Health, Farmington, CT; 3Trinity Health of New England, St. Francis Hospital, Hartford, CT
Introduction: Glucagon Like Peptide (GLP)-1 receptor agonists are a new class of antihyperglycemic agents which are being utilized in large numbers to treat Type 2 Diabetes Mellitus (T2DM) among other conditions. As mimetics of the endogenous secretagogue, these drugs potentiate insulin, stabilize post-prandial glucose, and importantly, delay gastric emptying. Patients with DM can develop delayed gastric emptying, dyspepsia, and frank gastroparesis through direct action of glucose on enteric motility and hyperglycemia-induced loss of neurons. There is little literature existing that examines symptom changes in this patient population at risk for delayed gastric emptying following treatment with this class of drugs. Here we report on a case series of 2 patients assessed with validated symptom rating scales over time.
Case Description/
Methods: Patients with T2DM were identified in the ambulatory setting who met criteria for treatment with GLP-1 agonists (patient demographics in Table 1). They were administered a battery of 3 surveys prior to treatment: the Gastroparesis Cardinal Symptom Index (GCSI), Patient Assessment of Upper GI Quality of Life (PAGI-QOL), Gastrointestinal Symptom Rating Scale (GSRS). Patient 1 is a 47-year old female with hypertension, history of HIV well-controlled with antiretrovirals, long-standing T2DM managed with insulin and oral agents who sought to start a GLP-1 agonist for increased glycemic control and weight loss. Patient 2 is a 33-year-old male with hypertension and bipolar 1 disorder who’s T2DM was managed with oral antihyperglycemic medications and was looking to start GLP-1 agonists for the added weight benefits. Both patients had poorly controlled diabetes at the initiation of the GLP-1 agonists, with A1c above 8%. They were initially screened for gastroparesis with the GCSI, which was negative. Initial composite GSRS scores were 14.7 and 28.2, respectively. Initial PAGI-QOL were high, indicating poor quality of life due to symptoms (113 and 50). Notably, there was improvement in both patients in overall PAGI-QOL, decreasing to 23 for Patient 1 and 17 for Patient 2. Composite GSRS was also decreased for both patients, 8.3 for Patient 1 and 1 for Patient 2.
Discussion: The unexpected response to these drugs highlights the variable effects they can have on dyspeptic symptoms in patients with T2DM. We continue to follow these patients to get a better sense of factors that may predict gastrointestinal effects of GLP-1 agonists in vivo.
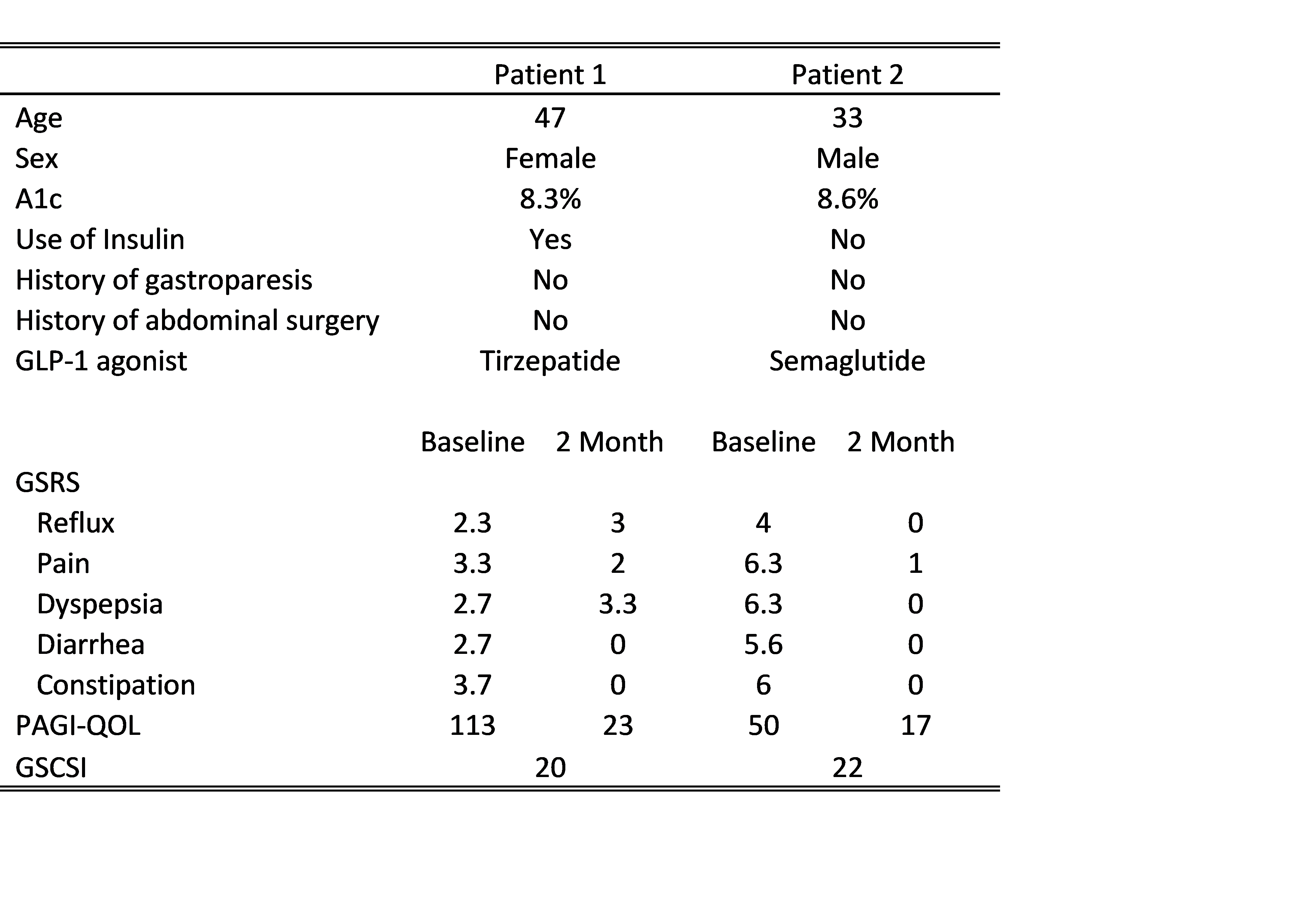
Figure: Table 1: Relevant patient information and survey scores from baseline to two month reassessment.
Disclosures:
Christopher Costa indicated no relevant financial relationships.
Hira Khan indicated no relevant financial relationships.
Lisa Weisinger indicated no relevant financial relationships.
Christopher Costa, MD1, Hira Khan, MD2, Lisa Weisinger, MD3. P0832 - GLP-1 Agonists and Dyspeptic Symptoms: A Case Series in the Ambulatory Setting, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1University of Connecticut Health, Rochester, MN; 2University of Connecticut Health, Farmington, CT; 3Trinity Health of New England, St. Francis Hospital, Hartford, CT
Introduction: Glucagon Like Peptide (GLP)-1 receptor agonists are a new class of antihyperglycemic agents which are being utilized in large numbers to treat Type 2 Diabetes Mellitus (T2DM) among other conditions. As mimetics of the endogenous secretagogue, these drugs potentiate insulin, stabilize post-prandial glucose, and importantly, delay gastric emptying. Patients with DM can develop delayed gastric emptying, dyspepsia, and frank gastroparesis through direct action of glucose on enteric motility and hyperglycemia-induced loss of neurons. There is little literature existing that examines symptom changes in this patient population at risk for delayed gastric emptying following treatment with this class of drugs. Here we report on a case series of 2 patients assessed with validated symptom rating scales over time.
Case Description/
Methods: Patients with T2DM were identified in the ambulatory setting who met criteria for treatment with GLP-1 agonists (patient demographics in Table 1). They were administered a battery of 3 surveys prior to treatment: the Gastroparesis Cardinal Symptom Index (GCSI), Patient Assessment of Upper GI Quality of Life (PAGI-QOL), Gastrointestinal Symptom Rating Scale (GSRS). Patient 1 is a 47-year old female with hypertension, history of HIV well-controlled with antiretrovirals, long-standing T2DM managed with insulin and oral agents who sought to start a GLP-1 agonist for increased glycemic control and weight loss. Patient 2 is a 33-year-old male with hypertension and bipolar 1 disorder who’s T2DM was managed with oral antihyperglycemic medications and was looking to start GLP-1 agonists for the added weight benefits. Both patients had poorly controlled diabetes at the initiation of the GLP-1 agonists, with A1c above 8%. They were initially screened for gastroparesis with the GCSI, which was negative. Initial composite GSRS scores were 14.7 and 28.2, respectively. Initial PAGI-QOL were high, indicating poor quality of life due to symptoms (113 and 50). Notably, there was improvement in both patients in overall PAGI-QOL, decreasing to 23 for Patient 1 and 17 for Patient 2. Composite GSRS was also decreased for both patients, 8.3 for Patient 1 and 1 for Patient 2.
Discussion: The unexpected response to these drugs highlights the variable effects they can have on dyspeptic symptoms in patients with T2DM. We continue to follow these patients to get a better sense of factors that may predict gastrointestinal effects of GLP-1 agonists in vivo.
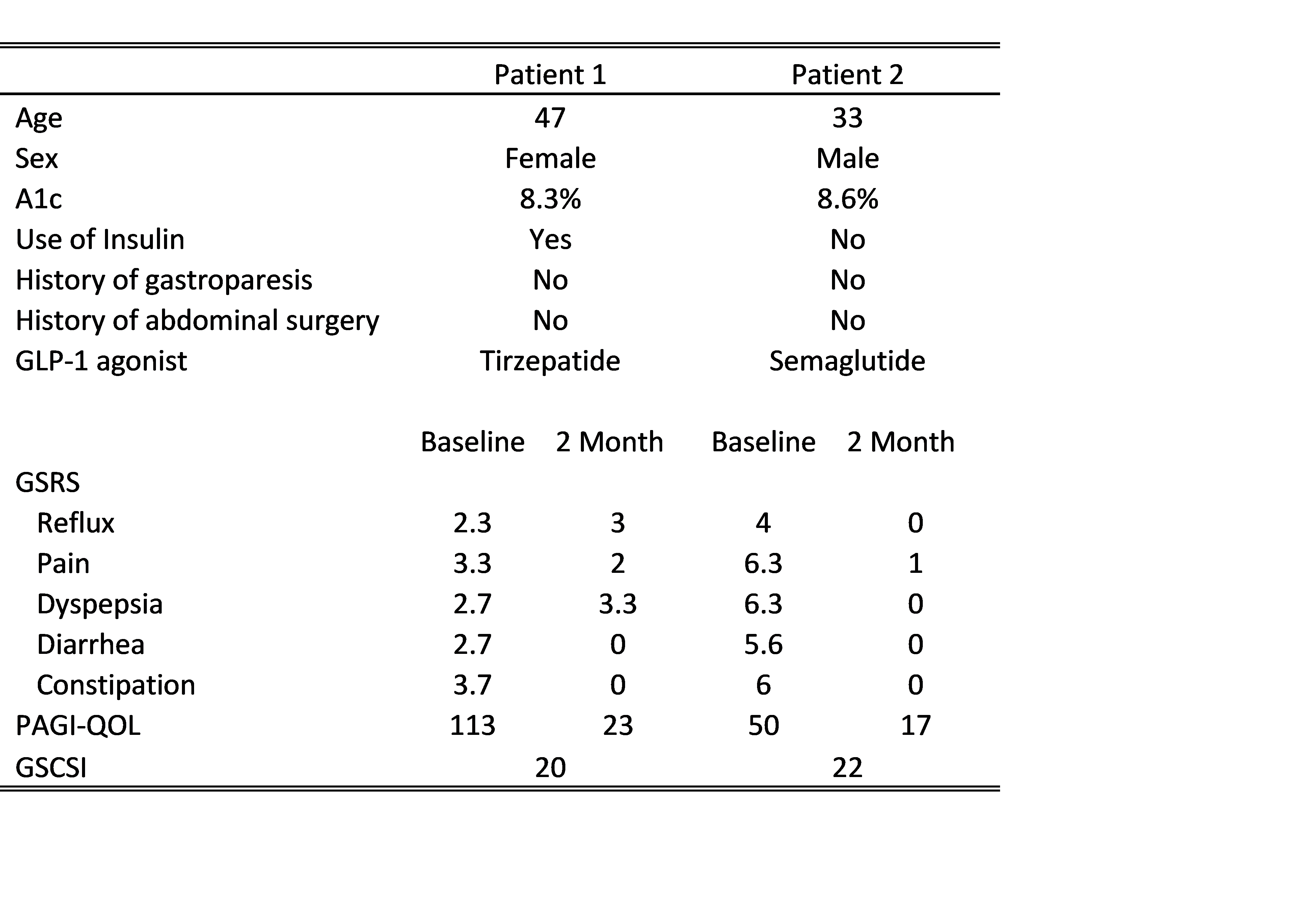
Figure: Table 1: Relevant patient information and survey scores from baseline to two month reassessment.
Disclosures:
Christopher Costa indicated no relevant financial relationships.
Hira Khan indicated no relevant financial relationships.
Lisa Weisinger indicated no relevant financial relationships.
Christopher Costa, MD1, Hira Khan, MD2, Lisa Weisinger, MD3. P0832 - GLP-1 Agonists and Dyspeptic Symptoms: A Case Series in the Ambulatory Setting, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
