Sunday Poster Session
Category: Functional Bowel Disease
P0829 - New Kid on the Block: Could Suzetrigine’s Promise of Pain Relief Improve Outcomes in Management of DGBI?
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall

Ellen C. Tan, DO
Allegheny Health Network
Pittsburgh, PA
Presenting Author(s)
Ellen C. Tan, DO1, Dayyan-Ur-Rahman Syed, 2, Saad Javed, MD1
1Allegheny Health Network, Pittsburgh, PA; 2University of Pittsburgh, Pittsburgh, PA
Introduction: Suzetrigine is a first-in-class, selective NaV1.8 sodium channel blocker expressed in peripheral sensory neurons, including dorsal root ganglion (DRG) neurons. Suzetrigine inhibits transmission of pain signals from peripheral sensory neurons to the spinal cord and brain. The U.S. Food and Drug Administration approved it on January 30, 2025 for the treatment of moderate-to-severe acute pain in adults. We describe two cases where suzetrigine was used for relief of abdominal pain.
Case Description/
Methods: 51-year-old man presents with chronic constipation associated with abdominal pain and bloating. Sitz marker study revealed delayed colonic transit. Colonoscopy, CT enterography and anorectal manometry were normal. He tried linaclotide, plecanatide, lubiprostone, prucalopride, and tenapanor. Tenapanor improved constipation and bloating, but he continued to have debilitating, episodic abdominal pain prompting several ER visits over three years. Adjunct pharmacological neuromodulators like nortriptyline, duloxetine and gabapentin were not helpful. A 14-day trial of suzetrigine (100mg initially, then 50mg twice daily) led to rapid and clinically meaningful pain relief.
21-year-old woman with history of anxiety developed recurrent, progressively worsening episodes of epigastric pain with no relation to bowel habits or food one year after a COVID-19 infection. Endoscopic evaluation, cross-sectional imaging, and gastric emptying study were normal. She responded partially to duloxetine, buspirone, mirtazapine, and cognitive behavioral therapy. After months of recurring symptoms and ER visits, suzetrigine allowed her to uptitrate augmentive neuromodulator therapy and achieve sustained pain relief.
Discussion: Pain-predominant disorders of gut-brain interaction (DGBI) have an unmet need for novel analgesics. There are limitations to the current standard of care with neuromodulators like TCAs and SNRIs due to inadequate pain relief or side effects. Suzetrigine’s inhibition of NaV1.8 reduces pain signals in primary human DRG sensory neurons with no adverse CNS, cardiovascular, or behavioral effects and no evidence of addictive potential or dependence based on nonclinical and clinical safety assessments. Studies have directly and indirectly established the importance of NaV1.8 to visceral pain perception (Table 1). Additional trials are needed to evaluate the impact of this medicine on visceral pain perception especially in conditions associated with acute and/or chronic abdominal pain, including DGBIs.
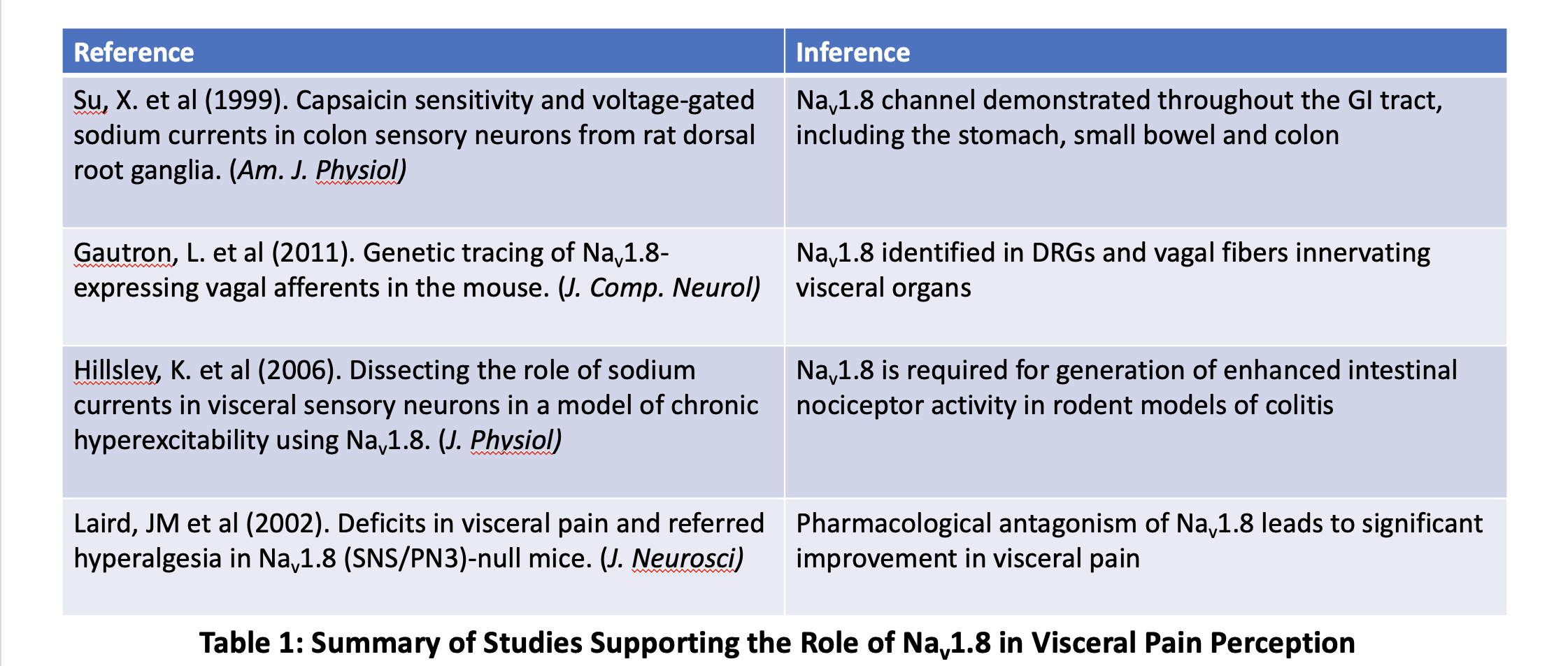
Figure: Summary of Studies Supporting the Role of Nav1.8 in Visceral Pain Perception
Disclosures:
Ellen Tan indicated no relevant financial relationships.
Dayyan-Ur-Rahman Syed indicated no relevant financial relationships.
Saad Javed indicated no relevant financial relationships.
Ellen C. Tan, DO1, Dayyan-Ur-Rahman Syed, 2, Saad Javed, MD1. P0829 - New Kid on the Block: Could Suzetrigine’s Promise of Pain Relief Improve Outcomes in Management of DGBI?, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Allegheny Health Network, Pittsburgh, PA; 2University of Pittsburgh, Pittsburgh, PA
Introduction: Suzetrigine is a first-in-class, selective NaV1.8 sodium channel blocker expressed in peripheral sensory neurons, including dorsal root ganglion (DRG) neurons. Suzetrigine inhibits transmission of pain signals from peripheral sensory neurons to the spinal cord and brain. The U.S. Food and Drug Administration approved it on January 30, 2025 for the treatment of moderate-to-severe acute pain in adults. We describe two cases where suzetrigine was used for relief of abdominal pain.
Case Description/
Methods: 51-year-old man presents with chronic constipation associated with abdominal pain and bloating. Sitz marker study revealed delayed colonic transit. Colonoscopy, CT enterography and anorectal manometry were normal. He tried linaclotide, plecanatide, lubiprostone, prucalopride, and tenapanor. Tenapanor improved constipation and bloating, but he continued to have debilitating, episodic abdominal pain prompting several ER visits over three years. Adjunct pharmacological neuromodulators like nortriptyline, duloxetine and gabapentin were not helpful. A 14-day trial of suzetrigine (100mg initially, then 50mg twice daily) led to rapid and clinically meaningful pain relief.
21-year-old woman with history of anxiety developed recurrent, progressively worsening episodes of epigastric pain with no relation to bowel habits or food one year after a COVID-19 infection. Endoscopic evaluation, cross-sectional imaging, and gastric emptying study were normal. She responded partially to duloxetine, buspirone, mirtazapine, and cognitive behavioral therapy. After months of recurring symptoms and ER visits, suzetrigine allowed her to uptitrate augmentive neuromodulator therapy and achieve sustained pain relief.
Discussion: Pain-predominant disorders of gut-brain interaction (DGBI) have an unmet need for novel analgesics. There are limitations to the current standard of care with neuromodulators like TCAs and SNRIs due to inadequate pain relief or side effects. Suzetrigine’s inhibition of NaV1.8 reduces pain signals in primary human DRG sensory neurons with no adverse CNS, cardiovascular, or behavioral effects and no evidence of addictive potential or dependence based on nonclinical and clinical safety assessments. Studies have directly and indirectly established the importance of NaV1.8 to visceral pain perception (Table 1). Additional trials are needed to evaluate the impact of this medicine on visceral pain perception especially in conditions associated with acute and/or chronic abdominal pain, including DGBIs.

Figure: Summary of Studies Supporting the Role of Nav1.8 in Visceral Pain Perception
Disclosures:
Ellen Tan indicated no relevant financial relationships.
Dayyan-Ur-Rahman Syed indicated no relevant financial relationships.
Saad Javed indicated no relevant financial relationships.
Ellen C. Tan, DO1, Dayyan-Ur-Rahman Syed, 2, Saad Javed, MD1. P0829 - New Kid on the Block: Could Suzetrigine’s Promise of Pain Relief Improve Outcomes in Management of DGBI?, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
