Sunday Poster Session
Category: Functional Bowel Disease
P0809 - Tenapanor Improves Abdominal Bloating Symptoms in Patients With IBS-C Experiencing Moderate to Severe Bloating
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall

Kyle Staller, MD, MPH
Massachusetts General Hospital
Boston, MA
Presenting Author(s)
Kyle Staller, MD, MPH1, Yang Yang, PhD2, Suling Zhao, 2, Susan Edelstein, PhD2
1Massachusetts General Hospital, Boston, MA; 2Ardelyx, Inc., Waltham, MA
Introduction: Abdominal bloating is one of the most bothersome symptoms of irritable bowel syndrome with constipation (IBS-C), yet it is not a typical primary endpoint in clinical trials. Tenapanor is a first-in-class, minimally systemic inhibitor of intestinal sodium-hydrogen exchanger isoform 3 approved by the US FDA for treatment of IBS-C in adults. Previous studies have demonstrated that tenapanor improves abdominal symptoms in IBS-C. This post hoc analysis assessed the effect of tenapanor on abdominal bloating in patients with IBS-C with moderate to severe bloating.
Methods: Data were pooled from a phase 2b (NCT01923428) and the phase 3 T3MPO-1 (NCT02621892) and T3MPO-2 (NCT02686138) studies, in which patients with IBS-C who met the Rome III criteria were randomized to tenapanor or placebo twice daily (bid) for 12 or 26 weeks. A phone diary was used to collect data on daily abdominal bloating on a scale of 0 to 10, categorized as < 4 (mild), ≥4 to < 8 (moderate), and ≥8 (severe). This analysis examined patients with moderate to severe bloating at baseline from the tenapanor 50 mg bid and placebo groups of the 3 studies (ie, the pooled efficacy analysis set). Efficacy endpoints included the change from baseline in average weekly bloating score and time to onset of achieving a ≥30% and ≥50% reduction from baseline in average weekly bloating score.
Results: In the pooled efficacy analysis set (n=1253), tenapanor treatment resulted in a consistently greater reduction from baseline in average weekly bloating score compared with placebo over the first 12 weeks, with a difference in least-squares mean change of 0.63 in week 8, and 0.56 in week 12 (Figure 1). There was a faster improvement in bloating among those treated with tenapanor compared with those treated with placebo. The median time to onset of achieving a ≥30% reduction in weekly bloating score was 5 weeks for tenapanor-treated patients versus 8 weeks for placebo-treated patients (log-rank test P<0.0001; Figure 2A). Median time to onset of achieving a ≥50% reduction in weekly bloating score is shown in Figure 2B.
Discussion: Tenapanor may be effective in reducing bloating–a persistent and bothersome symptom in a large percentage of patients with IBS-C. The onset of a clinically meaningful reduction can be as early as week 1 and sustains through the duration of treatment.
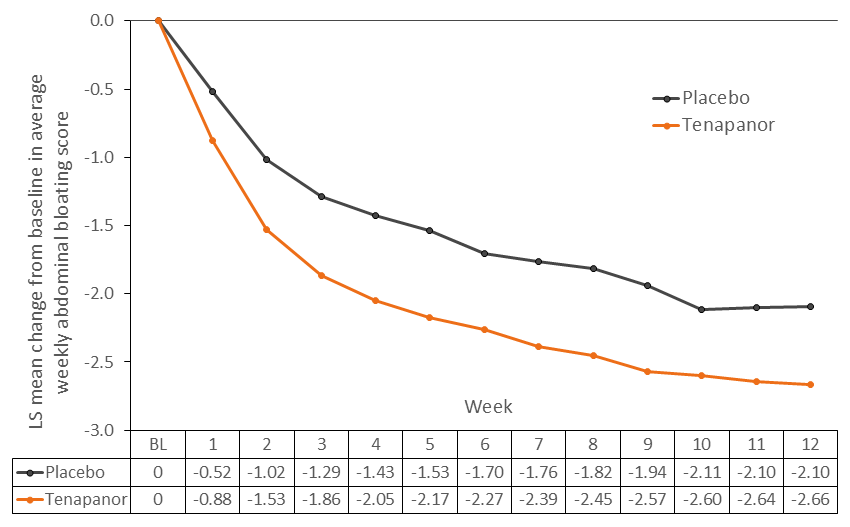
Figure: Figure 1: Least-Squares Mean Change (LS) From Baseline in Average Weekly Abdominal Bloating Score in Patients With Moderate to Severe Bloating at Baseline (BL).
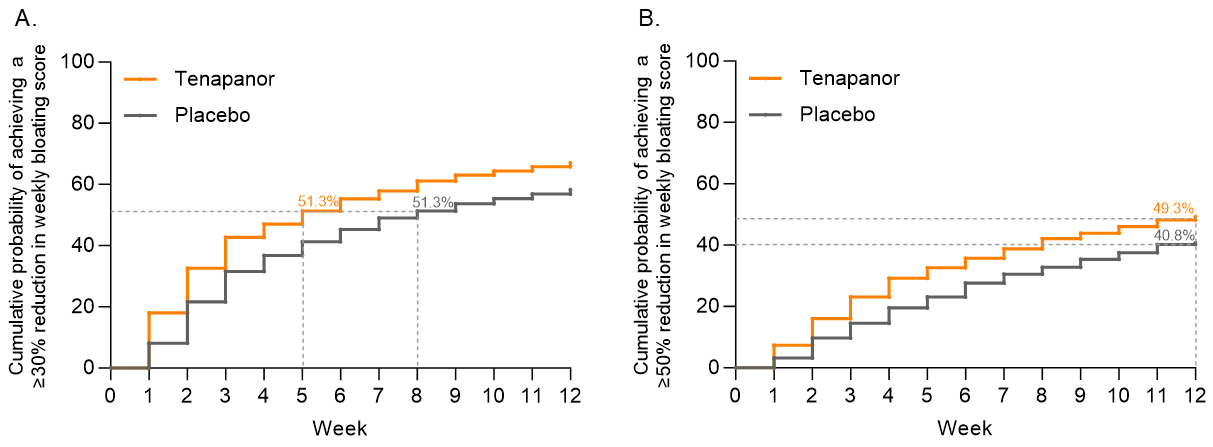
Figure: Figure 2: Time to Onset of Achieving a (A) ≥30% and (B) ≥50% Reduction in Weekly Bloating Score in Patients with Moderate to Severe Bloating at Baseline.
Disclosures:
Kyle Staller: Ardelyx – Grant/Research Support. Gemelli – Consultant. Laborie – Consultant. Mahana – Consultant. Salix – Consultant. Takeda – Expert witness.
Yang Yang: Ardelyx, Inc – Employee.
Suling Zhao: Ardelyx, Inc. – Employee.
Susan Edelstein: Ardelyx, Inc. – Employee.
Kyle Staller, MD, MPH1, Yang Yang, PhD2, Suling Zhao, 2, Susan Edelstein, PhD2. P0809 - Tenapanor Improves Abdominal Bloating Symptoms in Patients With IBS-C Experiencing Moderate to Severe Bloating, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Massachusetts General Hospital, Boston, MA; 2Ardelyx, Inc., Waltham, MA
Introduction: Abdominal bloating is one of the most bothersome symptoms of irritable bowel syndrome with constipation (IBS-C), yet it is not a typical primary endpoint in clinical trials. Tenapanor is a first-in-class, minimally systemic inhibitor of intestinal sodium-hydrogen exchanger isoform 3 approved by the US FDA for treatment of IBS-C in adults. Previous studies have demonstrated that tenapanor improves abdominal symptoms in IBS-C. This post hoc analysis assessed the effect of tenapanor on abdominal bloating in patients with IBS-C with moderate to severe bloating.
Methods: Data were pooled from a phase 2b (NCT01923428) and the phase 3 T3MPO-1 (NCT02621892) and T3MPO-2 (NCT02686138) studies, in which patients with IBS-C who met the Rome III criteria were randomized to tenapanor or placebo twice daily (bid) for 12 or 26 weeks. A phone diary was used to collect data on daily abdominal bloating on a scale of 0 to 10, categorized as < 4 (mild), ≥4 to < 8 (moderate), and ≥8 (severe). This analysis examined patients with moderate to severe bloating at baseline from the tenapanor 50 mg bid and placebo groups of the 3 studies (ie, the pooled efficacy analysis set). Efficacy endpoints included the change from baseline in average weekly bloating score and time to onset of achieving a ≥30% and ≥50% reduction from baseline in average weekly bloating score.
Results: In the pooled efficacy analysis set (n=1253), tenapanor treatment resulted in a consistently greater reduction from baseline in average weekly bloating score compared with placebo over the first 12 weeks, with a difference in least-squares mean change of 0.63 in week 8, and 0.56 in week 12 (Figure 1). There was a faster improvement in bloating among those treated with tenapanor compared with those treated with placebo. The median time to onset of achieving a ≥30% reduction in weekly bloating score was 5 weeks for tenapanor-treated patients versus 8 weeks for placebo-treated patients (log-rank test P<0.0001; Figure 2A). Median time to onset of achieving a ≥50% reduction in weekly bloating score is shown in Figure 2B.
Discussion: Tenapanor may be effective in reducing bloating–a persistent and bothersome symptom in a large percentage of patients with IBS-C. The onset of a clinically meaningful reduction can be as early as week 1 and sustains through the duration of treatment.

Figure: Figure 1: Least-Squares Mean Change (LS) From Baseline in Average Weekly Abdominal Bloating Score in Patients With Moderate to Severe Bloating at Baseline (BL).

Figure: Figure 2: Time to Onset of Achieving a (A) ≥30% and (B) ≥50% Reduction in Weekly Bloating Score in Patients with Moderate to Severe Bloating at Baseline.
Disclosures:
Kyle Staller: Ardelyx – Grant/Research Support. Gemelli – Consultant. Laborie – Consultant. Mahana – Consultant. Salix – Consultant. Takeda – Expert witness.
Yang Yang: Ardelyx, Inc – Employee.
Suling Zhao: Ardelyx, Inc. – Employee.
Susan Edelstein: Ardelyx, Inc. – Employee.
Kyle Staller, MD, MPH1, Yang Yang, PhD2, Suling Zhao, 2, Susan Edelstein, PhD2. P0809 - Tenapanor Improves Abdominal Bloating Symptoms in Patients With IBS-C Experiencing Moderate to Severe Bloating, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
