Sunday Poster Session
Category: Esophagus
P0598 - Long-Term Treatment With Dupilumab Maintains Histologic Improvements in Children With Eosinophilic Esophagitis (EoE): 100-Week Follow-Up Results From the EoE KIDS Study Open-Label Extension
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall

Margaret H. Collins, MD
Cincinnati Children’s Hospital Medical Center and University of Cincinnati College of Medicine
Cincinnati, OH
Presenting Author(s)
Award: ACG Presidential Poster Award
Margaret H. Collins, MD1, Marc E. Rothenberg, MD1, Diana Lerner, MD2, Robert D. Pesek, MD3, Navneet Virk Hundal, MD4, Ruiqi Liu, PhD5, Margee Louisias, MD, MPH6, Allen Radin, MD5
1Cincinnati Children’s Hospital Medical Center and University of Cincinnati College of Medicine, Cincinnati, OH; 2Medical College of Wisconsin, Milwaukee, WI; 3University of Arkansas for Medical Sciences and Arkansas Children’s Hospital, Little Rock, AR; 4Massachusetts General Hospital, Boston, MA; 5Regeneron Pharmaceuticals Inc., Tarrytown, NY; 6Sanofi, Cambridge, MA
Introduction: Eosinophilic esophagitis (EoE) is a chronic, progressive, type 2 inflammatory disease characterized by eosinophilic infiltration and other histopathologic abnormalities of the esophageal mucosa. Dupilumab is approved for EoE in the USA and EU in patients aged ≥1 year and weighing ≥15 kg. The phase 3 EoE KIDS trial (NCT04394351) evaluated efficacy and safety of dupilumab vs placebo in pediatric patients with active EoE. Here, we examined histopathologic features of EoE after dupilumab treatment in the open-label extension period of EoE KIDS.
Methods: In EoE KIDS, patients aged ≥1 to < 12 years were randomized to dupilumab or placebo for a 16-week double-blind period (Part A), switched to or continued active treatment for a 36-week extension (Part B), and then proceeded to an open-label extension (Part C) where all patients received the same weight-tiered dupilumab regimen. Esophageal biopsies were assessed for peak esophageal intraepithelial eosinophil count (PEC) and EoE Histologic Scoring System (EoEHSS) scores. The EoEHSS evaluates the severity (grade) and extent (stage) of 8 EoE features associated with inflammatory or architectural abnormalities; higher scores are associated with greater severity/extent of disease features.
Results: Overall, 61 patients were enrolled in Part C; 33 patients who previously received the higher-exposure dose of dupilumab (similar to the FDA-approved dose in EoE) were included in the analysis. Proportions of patients achieving histologic remission (PEC ≤6 eosinophils/high-power field [eos/hpf]) with dupilumab were maintained up to Week (W)100 (Figure). At W100 compared with W52, numerically greater proportions of the Part C population achieved PEC of ≤1 (45.5% vs 39.4%) and < 15 eos/hpf (90.9% vs 81.8%). Mean (95% confidence interval) changes from Part A baseline at W100 were maintained vs W52 in EoEHSS overall grade (−0.85 [−1.04 to −0.65] vs −0.87 [−1.01 to −0.73]) and stage scores (−0.87 [−1.04 to −0.70] vs −0.83 [−0.96 to −0.71]). Reductions in EoEHSS inflammatory and architectural grade/stage subscores were also maintained from W52 to W100.
Discussion: After 100 weeks of long-term treatment, dupilumab maintained or improved the proportions of pediatric patients with PEC ≤1, ≤6, and < 15 eos/hpf relative to W52. Long-term dupilumab also maintained improvements in the severity and extent of histopathologic features assessed by the EoEHSS.
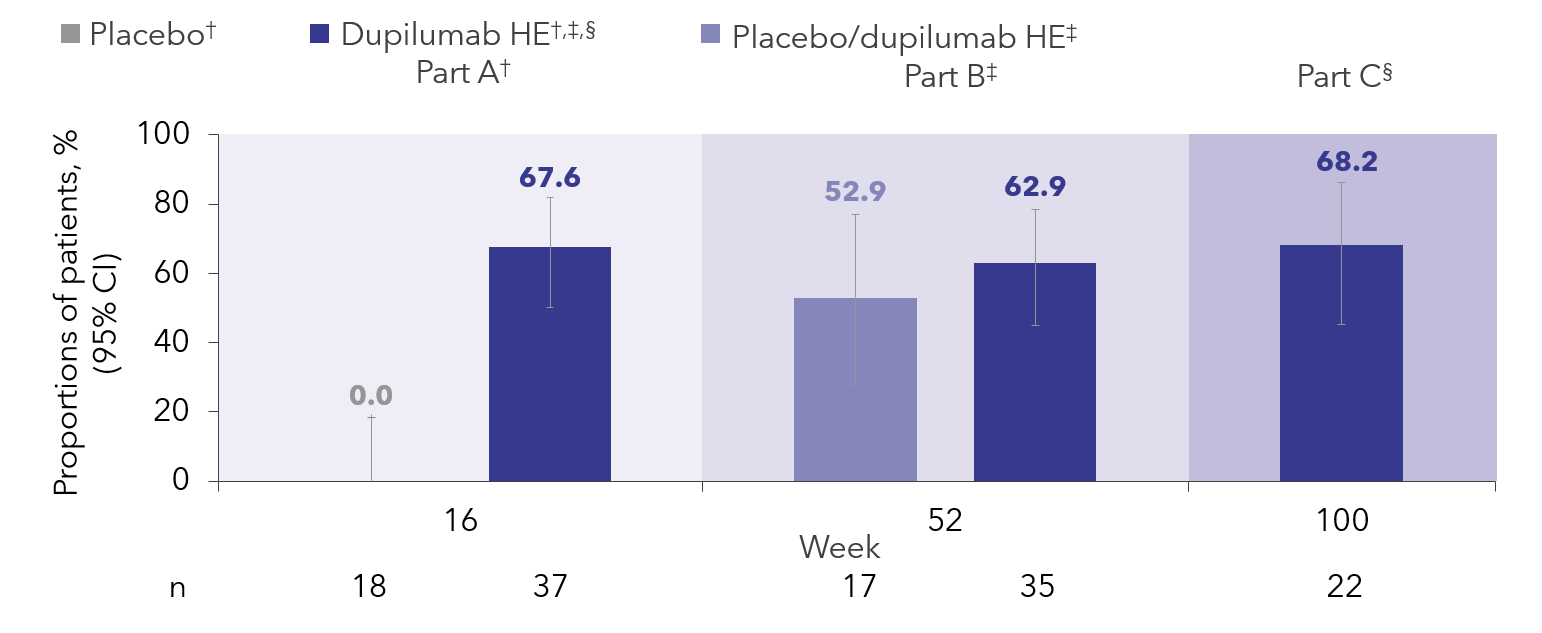
Figure: Figure. Proportions of patients achieving peak esophageal eosinophil count ≤6 eos/hpf during Parts A, B, and C of EoE KIDS.
† From W0 to W16, patients received either placebo or dupilumab HE (Part B safety population).
‡ From W16 to W52, the placebo/dupilumab HE and dupilumab HE/dupilumab HE groups received dupilumab HE (Part B safety population).
§ From W52 to W100, all patients received the dupilumab regimen later approved by the FDA for pediatric patients with EoE. This regimen was similar to the dupilumab HE regimen used in Parts A and B (Part C population).
CI, confidence interval; EoE, eosinophilic esophagitis; eos/hpf, eosinophils per high-power field; FDA, U.S. Food and Drug Administration; HE, higher exposure; W, Week.
Disclosures:
Margaret Collins: Alimentiv – Consultant. Arena/Pfizer – Consultant. AstraZeneca – Consultant. Calypso Biotech – Consultant. EsoCap Biotech – Consultant. GSK – Consultant. Receptos/Celgene/Bristol Myers Squibb – Consultant. Regeneron Pharmaceuticals Inc. – Consultant. Shire – Consultant.
Marc E. Rothenberg: Allakos – Advisory board/consulting fees, may hold stock and/or stock options. AstraZeneca – Advisory board/consulting fees, may hold stock and/or stock options. Biotherapeutics – Advisory board/consulting fees. Bristol Myers Squibb – Advisory board/consulting fees, may hold stock and/or stock options. Celldex – Advisory board/consulting fees, may hold stock and/or stock options. ClostraBio – Advisory board/consulting fees, may hold stock and/or stock options. EnZen Therapeutics – Advisory board/consulting fees, may hold stock and/or stock options. GSK – Advisory board/consulting fees, may hold stock and/or stock options. Guidepoint – Advisory board/consulting fees, may hold stock and/or stock options. Mapi Research Trust – Receives royalties from PEESSv2. Pfizer – Advisory board/consulting fees, may hold stock and/or stock options. PulmOne – Advisory board/consulting fees. Regeneron Pharmaceuticals Inc. – Advisory board/consulting fees. Sanofi – Advisory board/consulting fees. Santa Ana Bio – Advisory board/consulting fees. Serpin Pharma – Advisory board/consulting fees. Spoon Guru – Advisory board/consulting fees. Teva Pharmaceuticals – Receives royalties from reslizumab. Uniquitybio – Advisory board/consulting fees. UpToDate – Inventor of patents owned by Cincinnati children's hospital.
Diana Lerner: Lerner Media Inc. – Ownership interest. Regeneron Pharmaceuticals Inc. – Advisory board/consulting fees.
Robert Pesek: Regeneron Pharmaceuticals Inc. – Advisory board/consulting fees.
Navneet Virk Hundal: Aché – Advisory board/consulting fees. Danone – Advisory board/consulting fees. Nestlé – Advisory board/consulting fees. Sanofi – Advisory board/consulting fees.
Ruiqi Liu: Regeneron Pharmaceuticals Inc. – Employee and shareholder.
Margee Louisias: Sanofi – Employee, may hold stock and/or stock options in the company.
Allen Radin: Regeneron Pharmaceuticals Inc. – Employee and shareholder.
Margaret H. Collins, MD1, Marc E. Rothenberg, MD1, Diana Lerner, MD2, Robert D. Pesek, MD3, Navneet Virk Hundal, MD4, Ruiqi Liu, PhD5, Margee Louisias, MD, MPH6, Allen Radin, MD5. P0598 - Long-Term Treatment With Dupilumab Maintains Histologic Improvements in Children With Eosinophilic Esophagitis (EoE): 100-Week Follow-Up Results From the EoE KIDS Study Open-Label Extension, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
Margaret H. Collins, MD1, Marc E. Rothenberg, MD1, Diana Lerner, MD2, Robert D. Pesek, MD3, Navneet Virk Hundal, MD4, Ruiqi Liu, PhD5, Margee Louisias, MD, MPH6, Allen Radin, MD5
1Cincinnati Children’s Hospital Medical Center and University of Cincinnati College of Medicine, Cincinnati, OH; 2Medical College of Wisconsin, Milwaukee, WI; 3University of Arkansas for Medical Sciences and Arkansas Children’s Hospital, Little Rock, AR; 4Massachusetts General Hospital, Boston, MA; 5Regeneron Pharmaceuticals Inc., Tarrytown, NY; 6Sanofi, Cambridge, MA
Introduction: Eosinophilic esophagitis (EoE) is a chronic, progressive, type 2 inflammatory disease characterized by eosinophilic infiltration and other histopathologic abnormalities of the esophageal mucosa. Dupilumab is approved for EoE in the USA and EU in patients aged ≥1 year and weighing ≥15 kg. The phase 3 EoE KIDS trial (NCT04394351) evaluated efficacy and safety of dupilumab vs placebo in pediatric patients with active EoE. Here, we examined histopathologic features of EoE after dupilumab treatment in the open-label extension period of EoE KIDS.
Methods: In EoE KIDS, patients aged ≥1 to < 12 years were randomized to dupilumab or placebo for a 16-week double-blind period (Part A), switched to or continued active treatment for a 36-week extension (Part B), and then proceeded to an open-label extension (Part C) where all patients received the same weight-tiered dupilumab regimen. Esophageal biopsies were assessed for peak esophageal intraepithelial eosinophil count (PEC) and EoE Histologic Scoring System (EoEHSS) scores. The EoEHSS evaluates the severity (grade) and extent (stage) of 8 EoE features associated with inflammatory or architectural abnormalities; higher scores are associated with greater severity/extent of disease features.
Results: Overall, 61 patients were enrolled in Part C; 33 patients who previously received the higher-exposure dose of dupilumab (similar to the FDA-approved dose in EoE) were included in the analysis. Proportions of patients achieving histologic remission (PEC ≤6 eosinophils/high-power field [eos/hpf]) with dupilumab were maintained up to Week (W)100 (Figure). At W100 compared with W52, numerically greater proportions of the Part C population achieved PEC of ≤1 (45.5% vs 39.4%) and < 15 eos/hpf (90.9% vs 81.8%). Mean (95% confidence interval) changes from Part A baseline at W100 were maintained vs W52 in EoEHSS overall grade (−0.85 [−1.04 to −0.65] vs −0.87 [−1.01 to −0.73]) and stage scores (−0.87 [−1.04 to −0.70] vs −0.83 [−0.96 to −0.71]). Reductions in EoEHSS inflammatory and architectural grade/stage subscores were also maintained from W52 to W100.
Discussion: After 100 weeks of long-term treatment, dupilumab maintained or improved the proportions of pediatric patients with PEC ≤1, ≤6, and < 15 eos/hpf relative to W52. Long-term dupilumab also maintained improvements in the severity and extent of histopathologic features assessed by the EoEHSS.

Figure: Figure. Proportions of patients achieving peak esophageal eosinophil count ≤6 eos/hpf during Parts A, B, and C of EoE KIDS.
† From W0 to W16, patients received either placebo or dupilumab HE (Part B safety population).
‡ From W16 to W52, the placebo/dupilumab HE and dupilumab HE/dupilumab HE groups received dupilumab HE (Part B safety population).
§ From W52 to W100, all patients received the dupilumab regimen later approved by the FDA for pediatric patients with EoE. This regimen was similar to the dupilumab HE regimen used in Parts A and B (Part C population).
CI, confidence interval; EoE, eosinophilic esophagitis; eos/hpf, eosinophils per high-power field; FDA, U.S. Food and Drug Administration; HE, higher exposure; W, Week.
Disclosures:
Margaret Collins: Alimentiv – Consultant. Arena/Pfizer – Consultant. AstraZeneca – Consultant. Calypso Biotech – Consultant. EsoCap Biotech – Consultant. GSK – Consultant. Receptos/Celgene/Bristol Myers Squibb – Consultant. Regeneron Pharmaceuticals Inc. – Consultant. Shire – Consultant.
Marc E. Rothenberg: Allakos – Advisory board/consulting fees, may hold stock and/or stock options. AstraZeneca – Advisory board/consulting fees, may hold stock and/or stock options. Biotherapeutics – Advisory board/consulting fees. Bristol Myers Squibb – Advisory board/consulting fees, may hold stock and/or stock options. Celldex – Advisory board/consulting fees, may hold stock and/or stock options. ClostraBio – Advisory board/consulting fees, may hold stock and/or stock options. EnZen Therapeutics – Advisory board/consulting fees, may hold stock and/or stock options. GSK – Advisory board/consulting fees, may hold stock and/or stock options. Guidepoint – Advisory board/consulting fees, may hold stock and/or stock options. Mapi Research Trust – Receives royalties from PEESSv2. Pfizer – Advisory board/consulting fees, may hold stock and/or stock options. PulmOne – Advisory board/consulting fees. Regeneron Pharmaceuticals Inc. – Advisory board/consulting fees. Sanofi – Advisory board/consulting fees. Santa Ana Bio – Advisory board/consulting fees. Serpin Pharma – Advisory board/consulting fees. Spoon Guru – Advisory board/consulting fees. Teva Pharmaceuticals – Receives royalties from reslizumab. Uniquitybio – Advisory board/consulting fees. UpToDate – Inventor of patents owned by Cincinnati children's hospital.
Diana Lerner: Lerner Media Inc. – Ownership interest. Regeneron Pharmaceuticals Inc. – Advisory board/consulting fees.
Robert Pesek: Regeneron Pharmaceuticals Inc. – Advisory board/consulting fees.
Navneet Virk Hundal: Aché – Advisory board/consulting fees. Danone – Advisory board/consulting fees. Nestlé – Advisory board/consulting fees. Sanofi – Advisory board/consulting fees.
Ruiqi Liu: Regeneron Pharmaceuticals Inc. – Employee and shareholder.
Margee Louisias: Sanofi – Employee, may hold stock and/or stock options in the company.
Allen Radin: Regeneron Pharmaceuticals Inc. – Employee and shareholder.
Margaret H. Collins, MD1, Marc E. Rothenberg, MD1, Diana Lerner, MD2, Robert D. Pesek, MD3, Navneet Virk Hundal, MD4, Ruiqi Liu, PhD5, Margee Louisias, MD, MPH6, Allen Radin, MD5. P0598 - Long-Term Treatment With Dupilumab Maintains Histologic Improvements in Children With Eosinophilic Esophagitis (EoE): 100-Week Follow-Up Results From the EoE KIDS Study Open-Label Extension, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.

