Sunday Poster Session
Category: Diet, Nutrition, and Obesity
P0555 - Gastrointestinal Tolerability of Tirzepatide Compared With GLP-1 Receptor Agonists, Insulin and Placebo in Obesity: A Meta-Analysis of 11,000+ Subjects
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall

Maitri Shah, MD (she/her/hers)
University of Michigan Health - Sparrow
East Lansing, MI
Presenting Author(s)
Maitri Shah, MD1, Shivam Kalra, MBBS, MHA2, Dushyant S. Dahiya, MD3, Sidra Naz, MD, MPH4, Shreeya kumar. Adhikari, MD1, Richa Tikaria, MD5
1University of Michigan Health - Sparrow, Lansing, MI; 2Trident Medical Center, North Charleston, SC; 3University of Kansas School of Medicine, Kansas City, KS; 4University of Texas MD Anderson Cancer Center, Houston, TX; 5Michigan State University, Lansing, MI
Introduction: Dual GLP1/GIP and GLP1 receptor agonists (GLP1 RA) are cornerstone therapies for obesity and Type 2 diabetes (T2DM). Gastrointestinal adverse events (GI AEs) are a major limiting factor for utility, often leading to treatment discontinuation, reduced adherence, hospitalizations, and gastroenterology referrals. The frequency and severity of GI AEs remain under-characterized, prompting this meta-analysis to clarify their profiles for clinicians navigating GI tolerability challenges.
Methods: PubMed, Embase and clinicaltrials.gov databases were searched through March 2025 for RCT’s and observational studies evaluating once-weekly subcutaneous Tirzepatide (5–15 mg) versus GLP1 RA, basal insulin and placebo in adults with BMI ≥ 25 kg m⁻² and T2DM. Primary outcomes included nausea, vomiting, diarrhea, and constipation; secondary outcomes were abdominal pain and elevated lipase. Pooled odds ratios (OR) with 95 % confidence intervals (CI) were calculated using Random-effects models; I² assessed heterogeneity.
Results: Fourteen trials (N=11,502) were included. Mean age: 58.1 years; BMI: 32.7 kg/m²; weight: 90.5 kg; 54% men; baseline HbA1c: 8.4%. Tirzepatide was associated with higher GI AEs compared to insulin (nausea OR: 13.51, vomiting OR: 8.06, diarrhea OR: 4.77, constipation OR: 7.64) and placebo (nausea OR: 3.34, vomiting OR: 3.86, diarrhea OR: 2.30, constipation OR: 2.96). For secondary outcomes, Tirzepatide increased abdominal pain (OR: 3.20) and lipase (OR: 4.00) vs insulin. Compared to placebo, only lipase was elevated (OR: 1.99); abdominal pain showed no difference. No significant differences in primary and secondary outcomes were observed when compared to GLP1 RAs.
Discussion: In our meta-analysis, we found that the GI AE profile of Tirzepatide closely mirrors that of GLP1 RAs. Confirming that dual-agonist medications do not amplify GLP1-related GI intolerance and support Tirzepatide as a well-tolerated, weight-centric treatment for obesity-driven T2DM. However, the odds ratio remained notably higher with insulin compared to placebo. This elevated ratio likely reflects lower GI AEs with insulin, rather than intrinsic toxicity from Tirzepatide. Future patient-level analyses would help identify predictors of intolerance to optimize dosing and maximize therapeutic benefit. Furthermore, these AEs were dose-dependent, with higher doses correlating with increased frequency and severity. Hence, careful titration of medication in high-risk populations may enhance therapeutic outcomes.
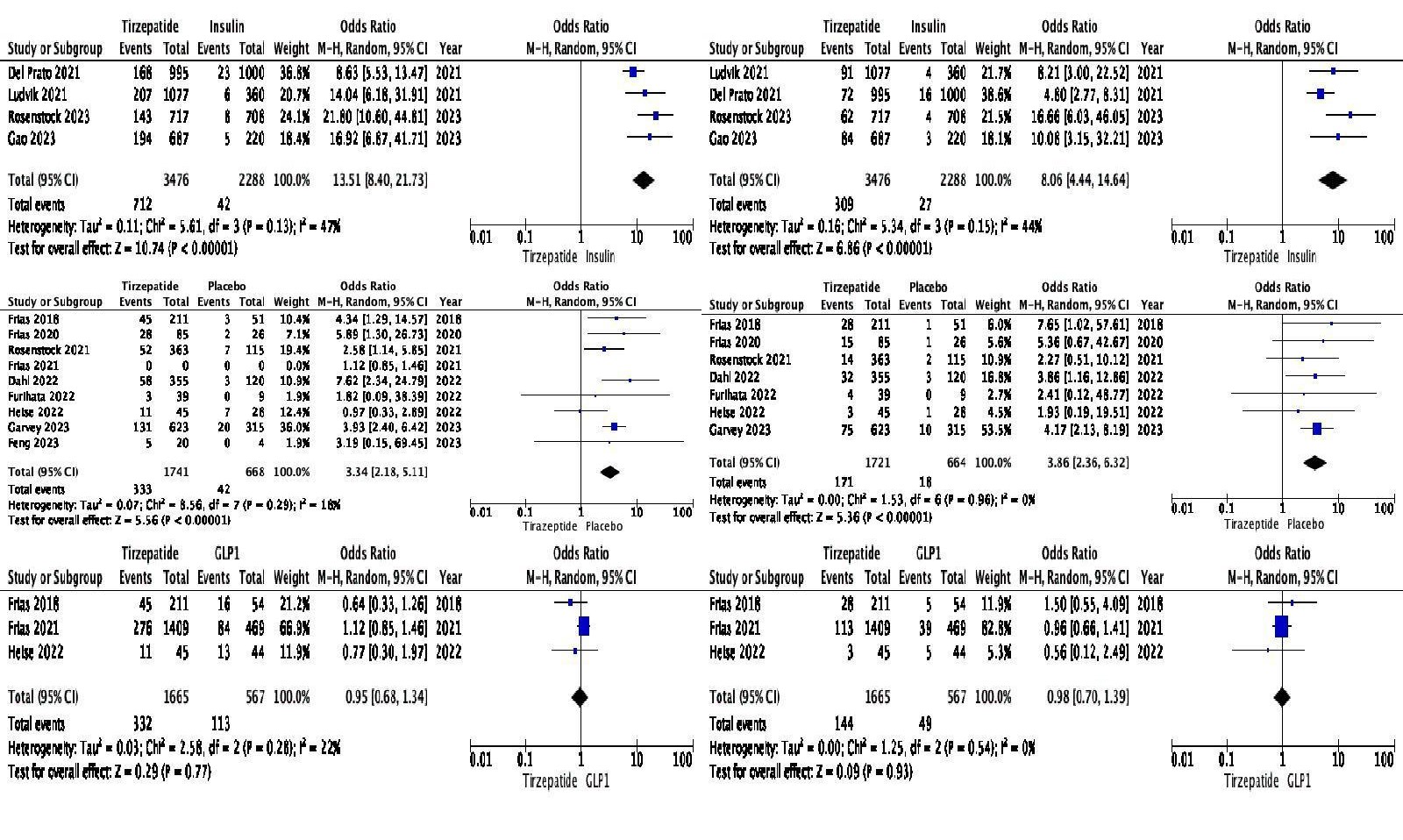
Figure: Pooled Odds Ratio Comparing Tirzepatide with Insulin, GLP1 and Placebo (Top to Bottom). Primary outcome Nausea (Left), Vomiting (Right)
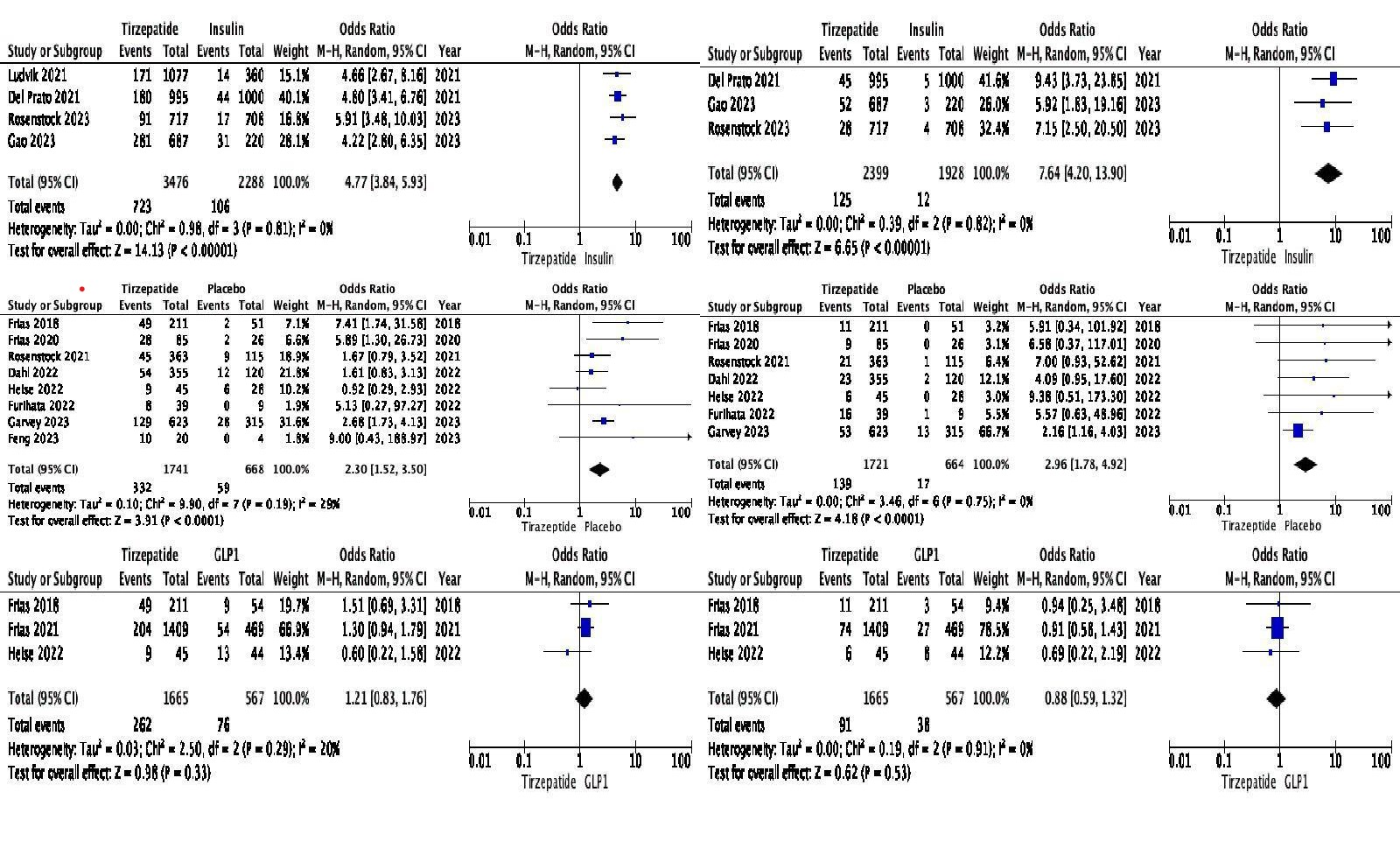
Figure: Pooled Odds Ratio Comparing Tirzepatide with Insulin, GLP1 and Placebo (Top to Bottom). Primary outcome Diarrhea (Left), Constipation (Right)
Disclosures:
Maitri Shah indicated no relevant financial relationships.
Shivam Kalra indicated no relevant financial relationships.
Dushyant Dahiya indicated no relevant financial relationships.
Sidra Naz indicated no relevant financial relationships.
Shreeya Adhikari indicated no relevant financial relationships.
Richa Tikaria indicated no relevant financial relationships.
Maitri Shah, MD1, Shivam Kalra, MBBS, MHA2, Dushyant S. Dahiya, MD3, Sidra Naz, MD, MPH4, Shreeya kumar. Adhikari, MD1, Richa Tikaria, MD5. P0555 - Gastrointestinal Tolerability of Tirzepatide Compared With GLP-1 Receptor Agonists, Insulin and Placebo in Obesity: A Meta-Analysis of 11,000+ Subjects, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1University of Michigan Health - Sparrow, Lansing, MI; 2Trident Medical Center, North Charleston, SC; 3University of Kansas School of Medicine, Kansas City, KS; 4University of Texas MD Anderson Cancer Center, Houston, TX; 5Michigan State University, Lansing, MI
Introduction: Dual GLP1/GIP and GLP1 receptor agonists (GLP1 RA) are cornerstone therapies for obesity and Type 2 diabetes (T2DM). Gastrointestinal adverse events (GI AEs) are a major limiting factor for utility, often leading to treatment discontinuation, reduced adherence, hospitalizations, and gastroenterology referrals. The frequency and severity of GI AEs remain under-characterized, prompting this meta-analysis to clarify their profiles for clinicians navigating GI tolerability challenges.
Methods: PubMed, Embase and clinicaltrials.gov databases were searched through March 2025 for RCT’s and observational studies evaluating once-weekly subcutaneous Tirzepatide (5–15 mg) versus GLP1 RA, basal insulin and placebo in adults with BMI ≥ 25 kg m⁻² and T2DM. Primary outcomes included nausea, vomiting, diarrhea, and constipation; secondary outcomes were abdominal pain and elevated lipase. Pooled odds ratios (OR) with 95 % confidence intervals (CI) were calculated using Random-effects models; I² assessed heterogeneity.
Results: Fourteen trials (N=11,502) were included. Mean age: 58.1 years; BMI: 32.7 kg/m²; weight: 90.5 kg; 54% men; baseline HbA1c: 8.4%. Tirzepatide was associated with higher GI AEs compared to insulin (nausea OR: 13.51, vomiting OR: 8.06, diarrhea OR: 4.77, constipation OR: 7.64) and placebo (nausea OR: 3.34, vomiting OR: 3.86, diarrhea OR: 2.30, constipation OR: 2.96). For secondary outcomes, Tirzepatide increased abdominal pain (OR: 3.20) and lipase (OR: 4.00) vs insulin. Compared to placebo, only lipase was elevated (OR: 1.99); abdominal pain showed no difference. No significant differences in primary and secondary outcomes were observed when compared to GLP1 RAs.
Discussion: In our meta-analysis, we found that the GI AE profile of Tirzepatide closely mirrors that of GLP1 RAs. Confirming that dual-agonist medications do not amplify GLP1-related GI intolerance and support Tirzepatide as a well-tolerated, weight-centric treatment for obesity-driven T2DM. However, the odds ratio remained notably higher with insulin compared to placebo. This elevated ratio likely reflects lower GI AEs with insulin, rather than intrinsic toxicity from Tirzepatide. Future patient-level analyses would help identify predictors of intolerance to optimize dosing and maximize therapeutic benefit. Furthermore, these AEs were dose-dependent, with higher doses correlating with increased frequency and severity. Hence, careful titration of medication in high-risk populations may enhance therapeutic outcomes.

Figure: Pooled Odds Ratio Comparing Tirzepatide with Insulin, GLP1 and Placebo (Top to Bottom). Primary outcome Nausea (Left), Vomiting (Right)

Figure: Pooled Odds Ratio Comparing Tirzepatide with Insulin, GLP1 and Placebo (Top to Bottom). Primary outcome Diarrhea (Left), Constipation (Right)
Disclosures:
Maitri Shah indicated no relevant financial relationships.
Shivam Kalra indicated no relevant financial relationships.
Dushyant Dahiya indicated no relevant financial relationships.
Sidra Naz indicated no relevant financial relationships.
Shreeya Adhikari indicated no relevant financial relationships.
Richa Tikaria indicated no relevant financial relationships.
Maitri Shah, MD1, Shivam Kalra, MBBS, MHA2, Dushyant S. Dahiya, MD3, Sidra Naz, MD, MPH4, Shreeya kumar. Adhikari, MD1, Richa Tikaria, MD5. P0555 - Gastrointestinal Tolerability of Tirzepatide Compared With GLP-1 Receptor Agonists, Insulin and Placebo in Obesity: A Meta-Analysis of 11,000+ Subjects, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
