Sunday Poster Session
Category: Diet, Nutrition, and Obesity
P0547 - Meta-Analysis of Survodutide: Impact on Satiety, Appetite, Gastrointestinal Adverse Effects, and Drug Discontinuation
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall

Adarsh Pendyala, MBBS (he/him/his)
University Hospitals Birmingham NHS Foundation Trust
Birmingham, England, United Kingdom
Presenting Author(s)
Smruti Karale, MBBS1, Sadaf Iftikhar, MBBS2, Adarsh Pendyala, MBBS3, Sweta Sahu, MBBS4, Sowmya Durga Subhasri Guna, MBBS5
1Government Medical College, Kolhapur, Maharashtra, Balitmore, MD; 2Akhtar Saeed Medical and dental college, Lahore, Punjab, Pakistan; 3University Hospitals Birmingham NHS Foundation Trust, Birmingham, England, United Kingdom; 4J.J.M. Medical College, Bhubaneswar, Orissa, India; 5Kamineni Academy of Medical Sciences, Hyderabad, Telangana, India
Introduction: Survodutide acts as a glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) dual receptor agonist, reducing appetite and promoting weight loss. Due to promising results, the FDA has expedited its development, and Phase 3 trials are underway for multiple indications. While these drugs represent a revolutionary advancement, gastrointestinal (GI) side effects are a major cause of drug discontinuation. This study examines Survodutide’s GI effects and their impact on adherence.
Methods: A systematic search in PubMed and Google Scholar for full-text articles published until May 2025, evaluating Survodutide's effect in treating diabetes, obesity, and MASH, was done. A meta-analysis using a random-effects model with Restricted Maximum Likelihood (REML) in R (metafor package) calculated odds ratios (OR), 95% confidence intervals (CI), heterogeneity (I²), and p-values. Outcomes included decreased appetite, early satiety, nausea, vomiting, diarrhea, constipation, abdominal pain, and drug discontinuation from GI side effects.
Results: A total of 1,225 patients from 5 RCTs were included, with 978 patients receiving Survodutide in different doses, and 247 in the control group. Survodutide-treated patients showed higher odds of decreased appetite (OR = 3.39, p = 0.0004, 95% CI: 0.54–1.90, I² = 19.6%). The odds of early satiety were higher but not statistically significant (OR = 3.37, p = 0.065, 95% CI: -0.08 to 2.51, I² = 0%). The odds of experiencing nausea (OR = 6.14, p < 0.001, 95% CI: 1.45–2.18, I² = 0%), vomiting (OR = 10.15, p < 0.001, 95% CI: 1.66–2.97, I² = 0%), and diarrhea (OR = 2.66, p < 0.001, 95% CI: 0.59–1.37, I² = 0%) were significantly higher in the Survodutide arm. Constipation (OR = 1.67, p = 0.368, 95% CI: -0.61 to 1.63, I² = 61.6%) and abdominal pain (OR = 3.11, p = 0.142, 95% CI: -0.38 to 2.65, I² = 0%) did not show statistically significant differences. Odds of treatment discontinuation due to gastrointestinal side effects were markedly elevated (OR = 9.24, p < 0.001, 95% CI: 1.14–3.31, I² = 0%).
Discussion: While showing potential for reducing appetite, Survodutide may increase the odds of nausea, vomiting, and diarrhea. Its poor gastrointestinal tolerability, which can lead to drug discontinuation, along with variability in dose-escalation and side-effect reporting, warrants further investigation. These findings underscore the need to enhance Survodutide's safety profile by optimizing its dosing regimen.
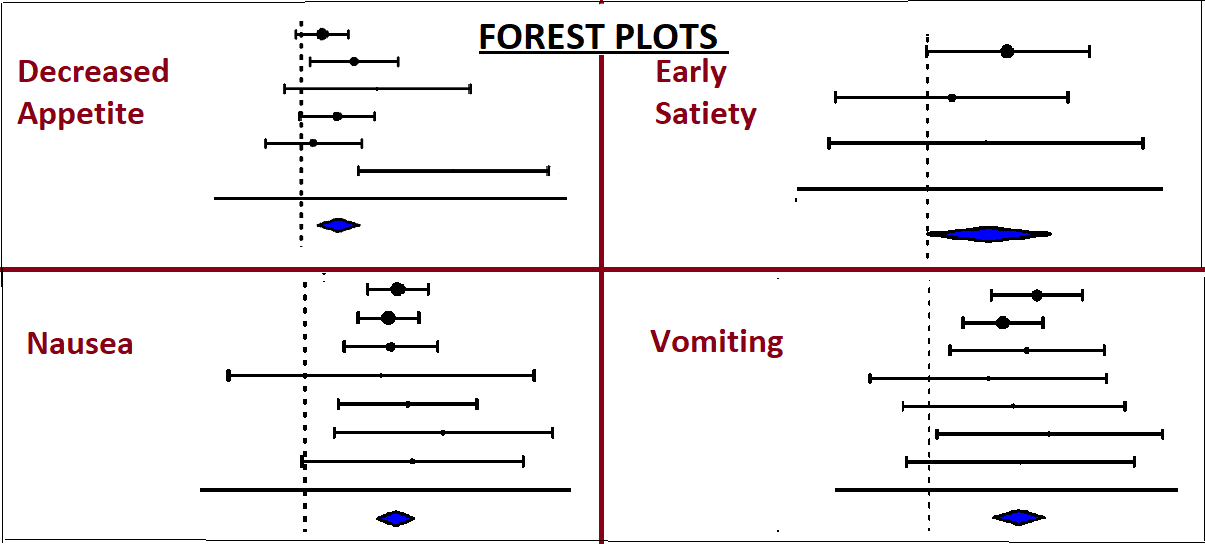
Figure: Side effects associated with Survodutide
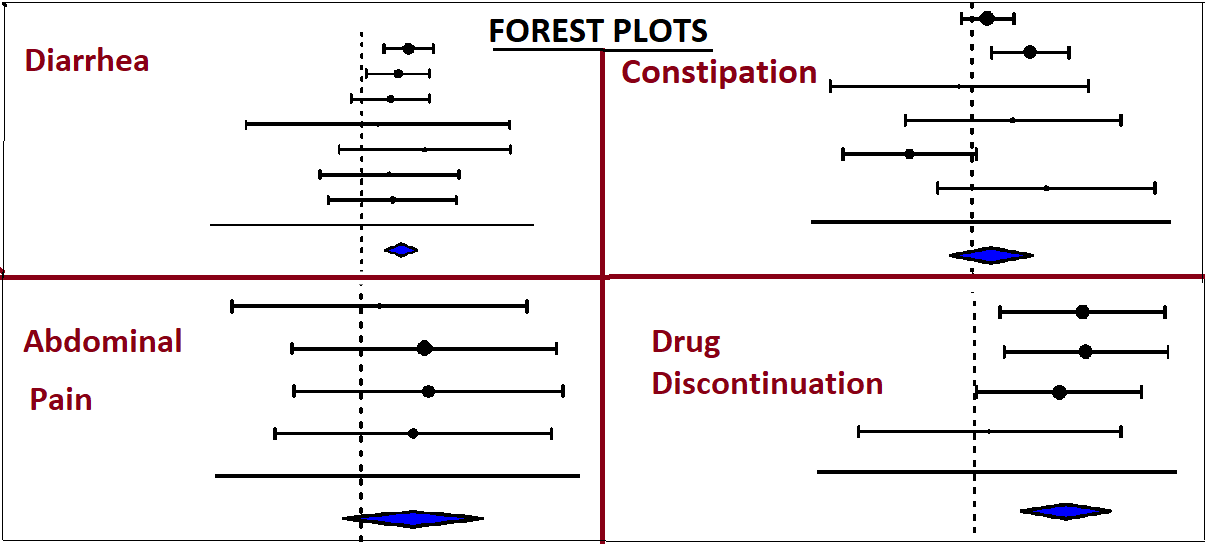
Figure: Side effects and impact on drug continuation
Disclosures:
Smruti Karale indicated no relevant financial relationships.
Sadaf Iftikhar indicated no relevant financial relationships.
Adarsh Pendyala indicated no relevant financial relationships.
Sweta Sahu indicated no relevant financial relationships.
Sowmya Durga Subhasri Guna indicated no relevant financial relationships.
Smruti Karale, MBBS1, Sadaf Iftikhar, MBBS2, Adarsh Pendyala, MBBS3, Sweta Sahu, MBBS4, Sowmya Durga Subhasri Guna, MBBS5. P0547 - Meta-Analysis of Survodutide: Impact on Satiety, Appetite, Gastrointestinal Adverse Effects, and Drug Discontinuation, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Government Medical College, Kolhapur, Maharashtra, Balitmore, MD; 2Akhtar Saeed Medical and dental college, Lahore, Punjab, Pakistan; 3University Hospitals Birmingham NHS Foundation Trust, Birmingham, England, United Kingdom; 4J.J.M. Medical College, Bhubaneswar, Orissa, India; 5Kamineni Academy of Medical Sciences, Hyderabad, Telangana, India
Introduction: Survodutide acts as a glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) dual receptor agonist, reducing appetite and promoting weight loss. Due to promising results, the FDA has expedited its development, and Phase 3 trials are underway for multiple indications. While these drugs represent a revolutionary advancement, gastrointestinal (GI) side effects are a major cause of drug discontinuation. This study examines Survodutide’s GI effects and their impact on adherence.
Methods: A systematic search in PubMed and Google Scholar for full-text articles published until May 2025, evaluating Survodutide's effect in treating diabetes, obesity, and MASH, was done. A meta-analysis using a random-effects model with Restricted Maximum Likelihood (REML) in R (metafor package) calculated odds ratios (OR), 95% confidence intervals (CI), heterogeneity (I²), and p-values. Outcomes included decreased appetite, early satiety, nausea, vomiting, diarrhea, constipation, abdominal pain, and drug discontinuation from GI side effects.
Results: A total of 1,225 patients from 5 RCTs were included, with 978 patients receiving Survodutide in different doses, and 247 in the control group. Survodutide-treated patients showed higher odds of decreased appetite (OR = 3.39, p = 0.0004, 95% CI: 0.54–1.90, I² = 19.6%). The odds of early satiety were higher but not statistically significant (OR = 3.37, p = 0.065, 95% CI: -0.08 to 2.51, I² = 0%). The odds of experiencing nausea (OR = 6.14, p < 0.001, 95% CI: 1.45–2.18, I² = 0%), vomiting (OR = 10.15, p < 0.001, 95% CI: 1.66–2.97, I² = 0%), and diarrhea (OR = 2.66, p < 0.001, 95% CI: 0.59–1.37, I² = 0%) were significantly higher in the Survodutide arm. Constipation (OR = 1.67, p = 0.368, 95% CI: -0.61 to 1.63, I² = 61.6%) and abdominal pain (OR = 3.11, p = 0.142, 95% CI: -0.38 to 2.65, I² = 0%) did not show statistically significant differences. Odds of treatment discontinuation due to gastrointestinal side effects were markedly elevated (OR = 9.24, p < 0.001, 95% CI: 1.14–3.31, I² = 0%).
Discussion: While showing potential for reducing appetite, Survodutide may increase the odds of nausea, vomiting, and diarrhea. Its poor gastrointestinal tolerability, which can lead to drug discontinuation, along with variability in dose-escalation and side-effect reporting, warrants further investigation. These findings underscore the need to enhance Survodutide's safety profile by optimizing its dosing regimen.

Figure: Side effects associated with Survodutide

Figure: Side effects and impact on drug continuation
Disclosures:
Smruti Karale indicated no relevant financial relationships.
Sadaf Iftikhar indicated no relevant financial relationships.
Adarsh Pendyala indicated no relevant financial relationships.
Sweta Sahu indicated no relevant financial relationships.
Sowmya Durga Subhasri Guna indicated no relevant financial relationships.
Smruti Karale, MBBS1, Sadaf Iftikhar, MBBS2, Adarsh Pendyala, MBBS3, Sweta Sahu, MBBS4, Sowmya Durga Subhasri Guna, MBBS5. P0547 - Meta-Analysis of Survodutide: Impact on Satiety, Appetite, Gastrointestinal Adverse Effects, and Drug Discontinuation, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
