Sunday Poster Session
Category: Colorectal Cancer Prevention
P0472 - Updated Quality Indicators for Colonoscopy in Patients with Positive Fecal Screening Tests
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall
- RM
Raakhi Menon, DO
University of Texas Medical Branch
Galveston, TX
Presenting Author(s)
Raakhi Menon, DO, Hari Movva, MD, Reema Patel, MD, Shayan Ravanassa, BS, Koushalya Sachdev, BS
University of Texas Medical Branch, Galveston, TX
Introduction: In August 2024, the American Journal of Gastroenterology released updated multisociety guidelines on colonoscopy quality indicators, including a 50% adenoma detection rate (ADR) benchmark for FIT and mt-sDNA positive patients and a longer withdrawal time (≥8 minutes). This study evaluates baseline ADR at our institution and the findings that may contribute to false positive screening tests.
Methods: We performed a retrospective review from August 2022 to August 2024 across two campuses, including patients aged ≥45 who had colonoscopy after positive FIT or mt-sDNA. Data included demographics, polyp characteristics, and hemorrhoid grading.
Results: Among 378 patients (284 mt-sDNA, 94 FIT), demographics were similar; mean age was ~63 years. Most were non-Hispanic (Cologuard: 90.5%, FIT: 80.9%).
ADR met or exceeded the 50% benchmark for both groups, with significantly higher rates in FIT (81.9%) vs. mt-sDNA (66.6%) (p = 0.0036). Tubular adenomas were the most common lesions in both cohorts. Large TAs ( >1 cm) were more frequent in mt-sDNA (22.2% vs. 20.2%), but not statistically significant (p=0.6781). SSAs were more prevalent with mt-sDNA (23.9% vs. 14.9%), trending toward significance (p= 0.0741). FIT had significantly more large TVAs (10.6% vs. 3.9%; p = 0.0069). HPs < 1 cm were more common in mt-sDNA (32.7% vs. 25.5%) though not statistically significant (p=0.2162). Cancer rates were similar (mt-sDNA: 3.5%; FIT: 5.3%; p=0.487).
False positives were more frequent with mt-sDNA (21.8% vs. 18.1%), though not significant (p=0.4524). Hemorrhoids were the most common alternate finding (grade ≥2: 30.7%; < grade 2: 38.7%).
Discussion: These findings support the new ADR benchmark for stool based colorectal cancer screening tests. While mt-sDNA detects a broader lesion range, FIT shows superior ADR and TVA detection. mt-sDNA’s broader sensitivity may reduce specificity due to benign findings like hemorrhoids and hyperplastic polyps.
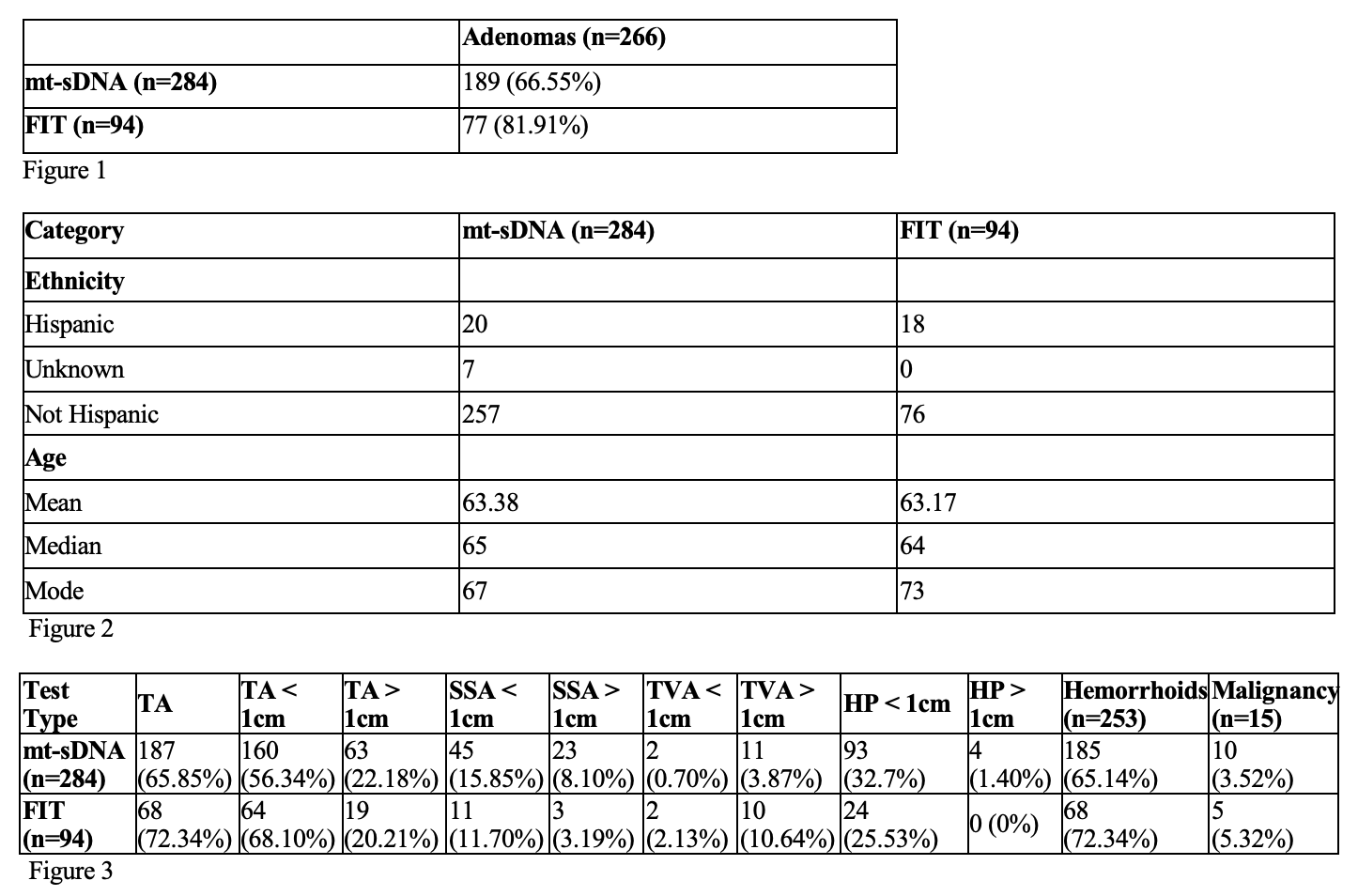
Figure: Figure 1: Number of Adenomas Based on Fecal Screening Test
Figure 2: Patient Demographics by Fecal Screening Test Type
Figure 3: Colonoscopy Findings by Fecal Screening Test Type
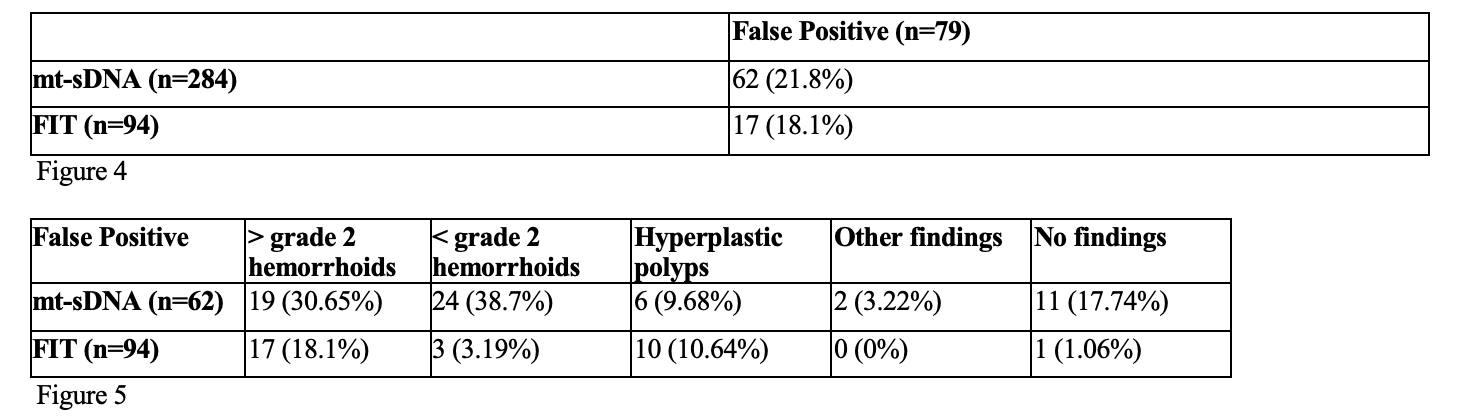
Figure: Figure 4: False Positive Rates by Fecal Screening Test Type
Figure 5: Endoscopic Findings in False Positive Cases
Disclosures:
Raakhi Menon indicated no relevant financial relationships.
Hari Movva indicated no relevant financial relationships.
Reema Patel indicated no relevant financial relationships.
Shayan Ravanassa indicated no relevant financial relationships.
Koushalya Sachdev indicated no relevant financial relationships.
Raakhi Menon, DO, Hari Movva, MD, Reema Patel, MD, Shayan Ravanassa, BS, Koushalya Sachdev, BS. P0472 - Updated Quality Indicators for Colonoscopy in Patients with Positive Fecal Screening Tests, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
University of Texas Medical Branch, Galveston, TX
Introduction: In August 2024, the American Journal of Gastroenterology released updated multisociety guidelines on colonoscopy quality indicators, including a 50% adenoma detection rate (ADR) benchmark for FIT and mt-sDNA positive patients and a longer withdrawal time (≥8 minutes). This study evaluates baseline ADR at our institution and the findings that may contribute to false positive screening tests.
Methods: We performed a retrospective review from August 2022 to August 2024 across two campuses, including patients aged ≥45 who had colonoscopy after positive FIT or mt-sDNA. Data included demographics, polyp characteristics, and hemorrhoid grading.
Results: Among 378 patients (284 mt-sDNA, 94 FIT), demographics were similar; mean age was ~63 years. Most were non-Hispanic (Cologuard: 90.5%, FIT: 80.9%).
ADR met or exceeded the 50% benchmark for both groups, with significantly higher rates in FIT (81.9%) vs. mt-sDNA (66.6%) (p = 0.0036). Tubular adenomas were the most common lesions in both cohorts. Large TAs ( >1 cm) were more frequent in mt-sDNA (22.2% vs. 20.2%), but not statistically significant (p=0.6781). SSAs were more prevalent with mt-sDNA (23.9% vs. 14.9%), trending toward significance (p= 0.0741). FIT had significantly more large TVAs (10.6% vs. 3.9%; p = 0.0069). HPs < 1 cm were more common in mt-sDNA (32.7% vs. 25.5%) though not statistically significant (p=0.2162). Cancer rates were similar (mt-sDNA: 3.5%; FIT: 5.3%; p=0.487).
False positives were more frequent with mt-sDNA (21.8% vs. 18.1%), though not significant (p=0.4524). Hemorrhoids were the most common alternate finding (grade ≥2: 30.7%; < grade 2: 38.7%).
Discussion: These findings support the new ADR benchmark for stool based colorectal cancer screening tests. While mt-sDNA detects a broader lesion range, FIT shows superior ADR and TVA detection. mt-sDNA’s broader sensitivity may reduce specificity due to benign findings like hemorrhoids and hyperplastic polyps.

Figure: Figure 1: Number of Adenomas Based on Fecal Screening Test
Figure 2: Patient Demographics by Fecal Screening Test Type
Figure 3: Colonoscopy Findings by Fecal Screening Test Type

Figure: Figure 4: False Positive Rates by Fecal Screening Test Type
Figure 5: Endoscopic Findings in False Positive Cases
Disclosures:
Raakhi Menon indicated no relevant financial relationships.
Hari Movva indicated no relevant financial relationships.
Reema Patel indicated no relevant financial relationships.
Shayan Ravanassa indicated no relevant financial relationships.
Koushalya Sachdev indicated no relevant financial relationships.
Raakhi Menon, DO, Hari Movva, MD, Reema Patel, MD, Shayan Ravanassa, BS, Koushalya Sachdev, BS. P0472 - Updated Quality Indicators for Colonoscopy in Patients with Positive Fecal Screening Tests, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
