Sunday Poster Session
Category: Colon
P0312 - A Post-Marketing Analysis of Gastrointestinal Adverse Events Following Chimeric Antigen Receptor T-Cell Therapy Using the FDA Adverse Event Reporting System
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall
- MG
Malvika Gupta, MD (she/her/hers)
Mayo Clinic, Rochester MN
Rochester, MN
Presenting Author(s)
Malvika Gupta, MD1, Supriya Gupta, MD2
1Mayo Clinic, Rochester MN, Rochester, MN; 2University of Minnesota, Minneapolis, MN
Introduction: Chimeric Antigen Receptor T-Cell (CAR-T) therapy has transformed the management of various cancers, with multiple products approved by the US Food and Drug Administration (FDA). However, CAR-T is associated with unique toxicities. Emerging studies show a link between CAR-T and gastrointestinal (GI) adverse events (AEs). As the use of CAR-T expands to a broader population, understanding its GI safety profile is critical.
Methods: We conducted a pharmacovigilance analysis of GI AEs reported to the FDA Adverse Event Reporting System (FAERS) for each CAR-T product through March 2025. Individual case safety reports listing any CAR-T therapies—Tisagenlecleucel (tisacel), Axicabtagene Ciloleucel (axicel), Lisocabtagene Maraleucel (lisocel), Brexucabtagene Autoleucel (brexucel), Idecabtagene Vicleucel (idecel), and Ciltacabtagene Autoleucel (ciltacel)—as the suspected drug for GI AEs were included. Patient characteristics were summarized, and disproportionality analysis with reported odds ratio was performed.
Results: There were 1395 GI AEs with CAR-T reported in FAERS (Table 1). Compared to other CAR-T, Ciltacel was associated with immune effector-cell mediated enterocolitis (IEC-EC) and non-immune colitis; Lisocel with GI ulceration, necrosis, and cholestasis; Idecel with functional/motility disorders; and Axicel and Tisacel with GI bleed. Tisacel was also associated with abnormal liver function tests, pancreatitis, and functional/motility disorders.
Distinct patterns were observed based on primary cancer. Aggressive B-cell lymphoma was associated with cholecystitis, bowel obstruction, and ascites; primary mediastinal large B-cell lymphoma with esophagitis and cholestasis; diffuse large B-cell lymphoma with gastroesophageal reflux and GI bleed; and B-acute lymphoblastic leukemia with pancreatitis.
Age and sex also influenced toxicity profile. Patients < 18 years had higher odds of developing pancreatitis and GI bleed. Patients >65 years were more likely to develop GI ulceration, functional/motility disorders, and non-immune colitis. Females were more likely to develop IEC-EC and non-immune colitis. Table 2 describes the significant associations.
Discussion: This post-marketing pharmacovigilance analysis highlights product and disease-specific patterns of GI AEs following CAR-T therapy. While limited by FAERS reporting bias, the findings support the need for monitoring strategies and emphasize the importance of prospective validation of these associations and to elucidate underlying mechanisms.
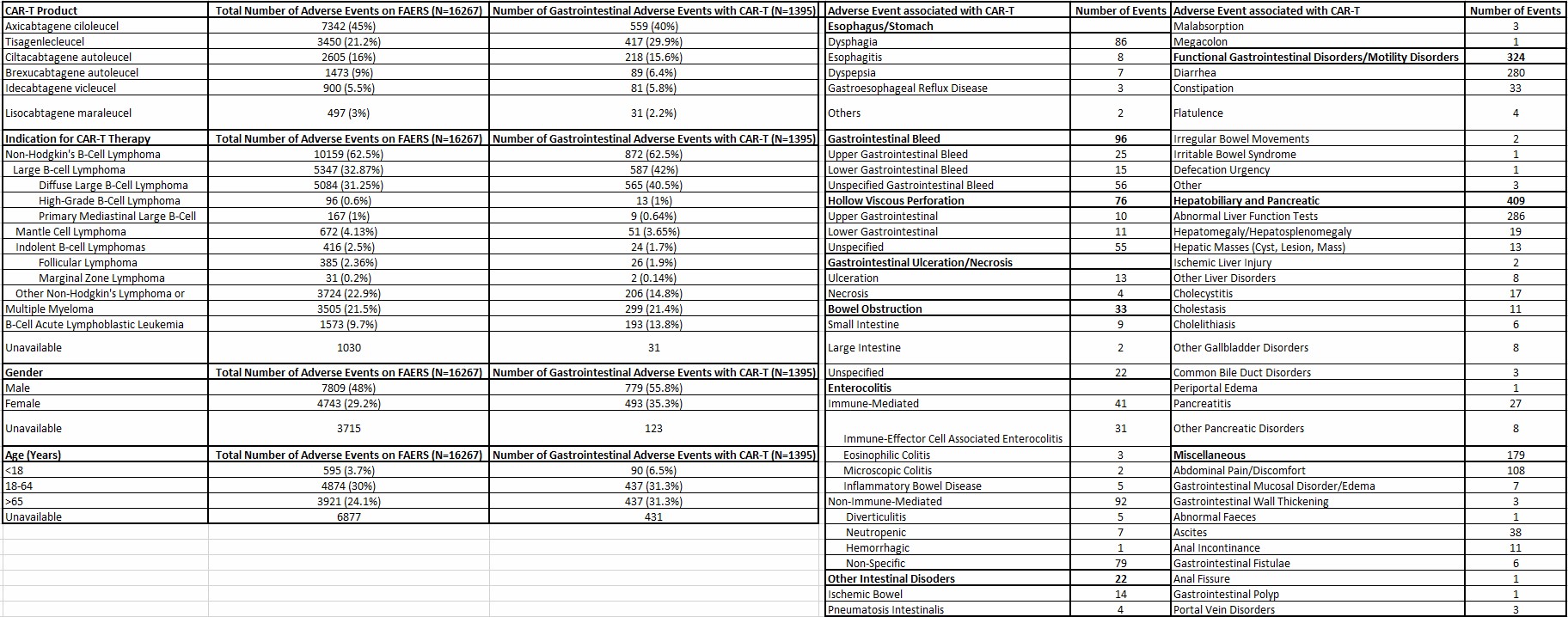
Figure: Table 1: Baseline Characteristics of Patients with Gastrointestinal Adverse Events with CAR-T Reported to the FDA Adverse Event Reporting System (FAERS)
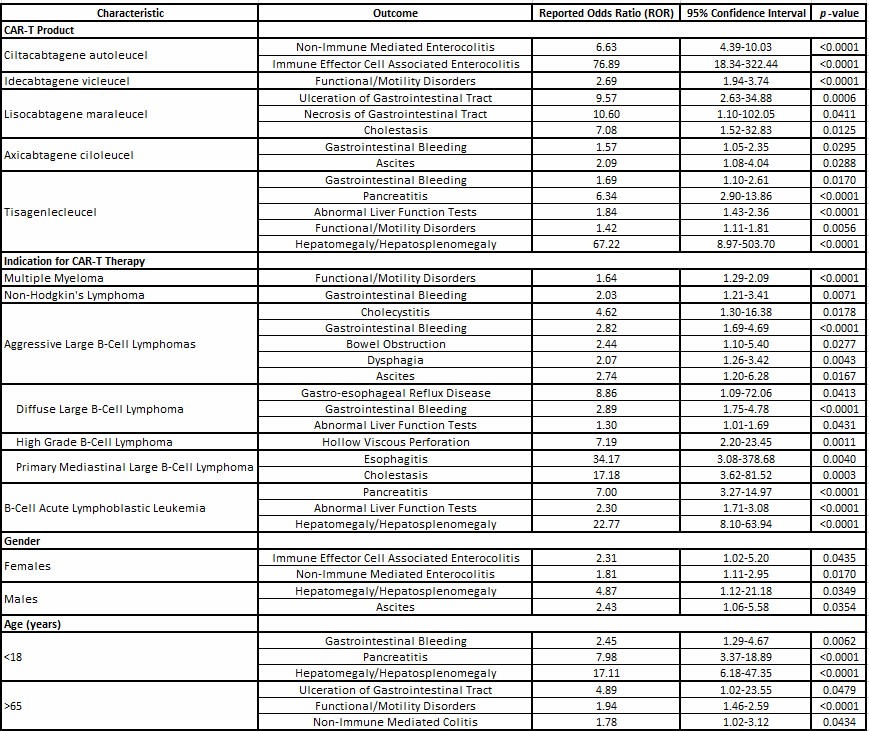
Figure: Table 2: Gastrointestinal Adverse Events Following CAR-T Therapy
Disclosures:
Malvika Gupta indicated no relevant financial relationships.
Supriya Gupta: Atara Biotherapeutics, INC. – Grant/Research Support. Imugene, INC. – Grant/Research Support.
Malvika Gupta, MD1, Supriya Gupta, MD2. P0312 - A Post-Marketing Analysis of Gastrointestinal Adverse Events Following Chimeric Antigen Receptor T-Cell Therapy Using the FDA Adverse Event Reporting System, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Mayo Clinic, Rochester MN, Rochester, MN; 2University of Minnesota, Minneapolis, MN
Introduction: Chimeric Antigen Receptor T-Cell (CAR-T) therapy has transformed the management of various cancers, with multiple products approved by the US Food and Drug Administration (FDA). However, CAR-T is associated with unique toxicities. Emerging studies show a link between CAR-T and gastrointestinal (GI) adverse events (AEs). As the use of CAR-T expands to a broader population, understanding its GI safety profile is critical.
Methods: We conducted a pharmacovigilance analysis of GI AEs reported to the FDA Adverse Event Reporting System (FAERS) for each CAR-T product through March 2025. Individual case safety reports listing any CAR-T therapies—Tisagenlecleucel (tisacel), Axicabtagene Ciloleucel (axicel), Lisocabtagene Maraleucel (lisocel), Brexucabtagene Autoleucel (brexucel), Idecabtagene Vicleucel (idecel), and Ciltacabtagene Autoleucel (ciltacel)—as the suspected drug for GI AEs were included. Patient characteristics were summarized, and disproportionality analysis with reported odds ratio was performed.
Results: There were 1395 GI AEs with CAR-T reported in FAERS (Table 1). Compared to other CAR-T, Ciltacel was associated with immune effector-cell mediated enterocolitis (IEC-EC) and non-immune colitis; Lisocel with GI ulceration, necrosis, and cholestasis; Idecel with functional/motility disorders; and Axicel and Tisacel with GI bleed. Tisacel was also associated with abnormal liver function tests, pancreatitis, and functional/motility disorders.
Distinct patterns were observed based on primary cancer. Aggressive B-cell lymphoma was associated with cholecystitis, bowel obstruction, and ascites; primary mediastinal large B-cell lymphoma with esophagitis and cholestasis; diffuse large B-cell lymphoma with gastroesophageal reflux and GI bleed; and B-acute lymphoblastic leukemia with pancreatitis.
Age and sex also influenced toxicity profile. Patients < 18 years had higher odds of developing pancreatitis and GI bleed. Patients >65 years were more likely to develop GI ulceration, functional/motility disorders, and non-immune colitis. Females were more likely to develop IEC-EC and non-immune colitis. Table 2 describes the significant associations.
Discussion: This post-marketing pharmacovigilance analysis highlights product and disease-specific patterns of GI AEs following CAR-T therapy. While limited by FAERS reporting bias, the findings support the need for monitoring strategies and emphasize the importance of prospective validation of these associations and to elucidate underlying mechanisms.

Figure: Table 1: Baseline Characteristics of Patients with Gastrointestinal Adverse Events with CAR-T Reported to the FDA Adverse Event Reporting System (FAERS)

Figure: Table 2: Gastrointestinal Adverse Events Following CAR-T Therapy
Disclosures:
Malvika Gupta indicated no relevant financial relationships.
Supriya Gupta: Atara Biotherapeutics, INC. – Grant/Research Support. Imugene, INC. – Grant/Research Support.
Malvika Gupta, MD1, Supriya Gupta, MD2. P0312 - A Post-Marketing Analysis of Gastrointestinal Adverse Events Following Chimeric Antigen Receptor T-Cell Therapy Using the FDA Adverse Event Reporting System, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
