Sunday Poster Session
Category: Colon
P0267 - Hypomotility of Lower Gastrointestinal Tract Post Immune Checkpoint Inhibitors
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall
- RM
Raakhi Menon, DO
University of Texas Medical Branch
Galveston, TX
Presenting Author(s)
Raakhi Menon, DO1, Sharada Wali, MBBS, MPH2, Rohan Ahuja, MD3, Samuel Black, MD4, Anusha Shirwaikar Thomas, MD2, Lavanya Viswanathan, MD, MS, FACG5, Mehnaz Shafi, MD2, Yinghong Wang, MD, PhD, MS2
1University of Texas Medical Branch, Galveston, TX; 2University of Texas MD Anderson Cancer Center, Houston, TX; 3University of Texas at Houston, Houston, TX; 4Baylor College of Medicine, Houston, TX; 5UT MD Anderson Cancer Center, Houston, TX
Introduction: Immune checkpoint inhibitors (ICIs) have transformed cancer therapy; however, their increased use has led to a rise in immune-related adverse events (irAEs). While diarrhea and colitis are well-documented gastrointestinal (GI) irAEs, emerging case studies now highlight ICI-related GI hypomotility. Constipation, though not typically classified as a GI irAE, is underreported in clinical practice, emphasizing the urgent need for further research.
Methods: This retrospective study analyzed adult patients with malignancies who developed constipation while receiving ICI therapy at a tertiary cancer center between 01/2010 and 02/2024 with deemed etiology related to ICI. Patients were excluded if symptoms were present prior to ICI therapy or attributed to other causes such as medications, comorbidities, surgeries, or mechanical obstructions. Clinical data and treatments as well as adverse events were reported.
Results: 72 patients were included, with a median age of 61 years (52-70), 59.7% male, and 70.8% Caucasian. Melanoma was the most common malignancy (34.7%), followed by hematological cancers (23.6%). Most patients (80.6%) received PD-1/L1 inhibitors. The median symptom duration was 20 days (8-48), with symptom onset occurring 96 days (34-172) post-ICI initiation. ICI was held in 2.8% of cases, and 12.5% required ER visits or hospitalization. Of note, 10 patients had both constipation and upper GI symptoms such as nausea/vomiting, with suspicion of a generalized motility disturbance. One patient underwent a colonoscopy, the pathology of which yielded non-specific findings.
Medical treatment for constipation was administered in 90.3% of cases, with complications occurring in 5.6%, including partial small bowel obstruction. Symptoms improved in 78.5% of patients, but 40.3% experienced recurrence within one month, and ICI was resumed in 55.6% of cases. Subgroup analysis showed that treatment responders (78.5%) had a median symptom duration of 24 days versus 105 days for non-responders. ICI was held in 2% of responders and 7.1% of non-responders. Recurrence rates after ICI resumption were much higher at 80.8% and 83.3%, respectively.
Discussion: While colitis is the most common gastrointestinal irAE, a subset of patients receiving ICI therapy may develop constipation. These findings suggest constipation may represent a less common but noteworthy adverse effect of ICI.
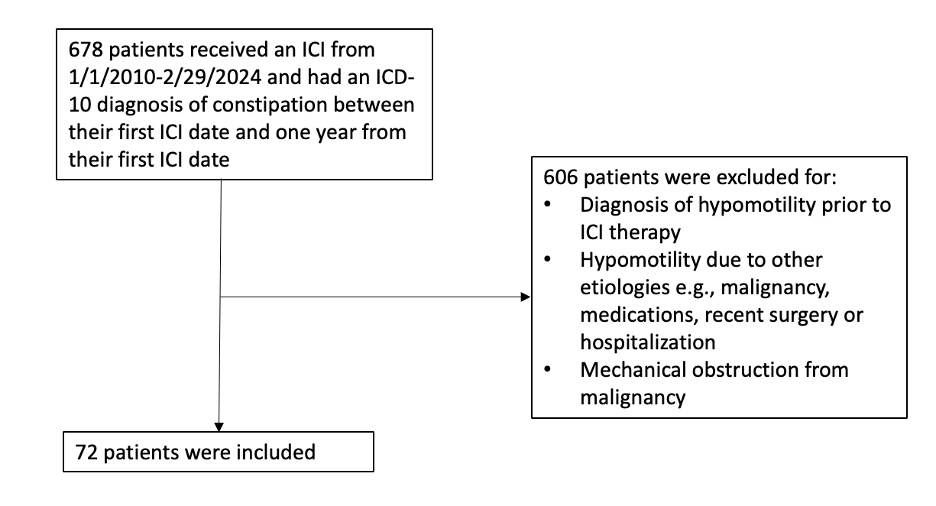
Figure: Figure 1. Inclusion and Exclusion Criteria
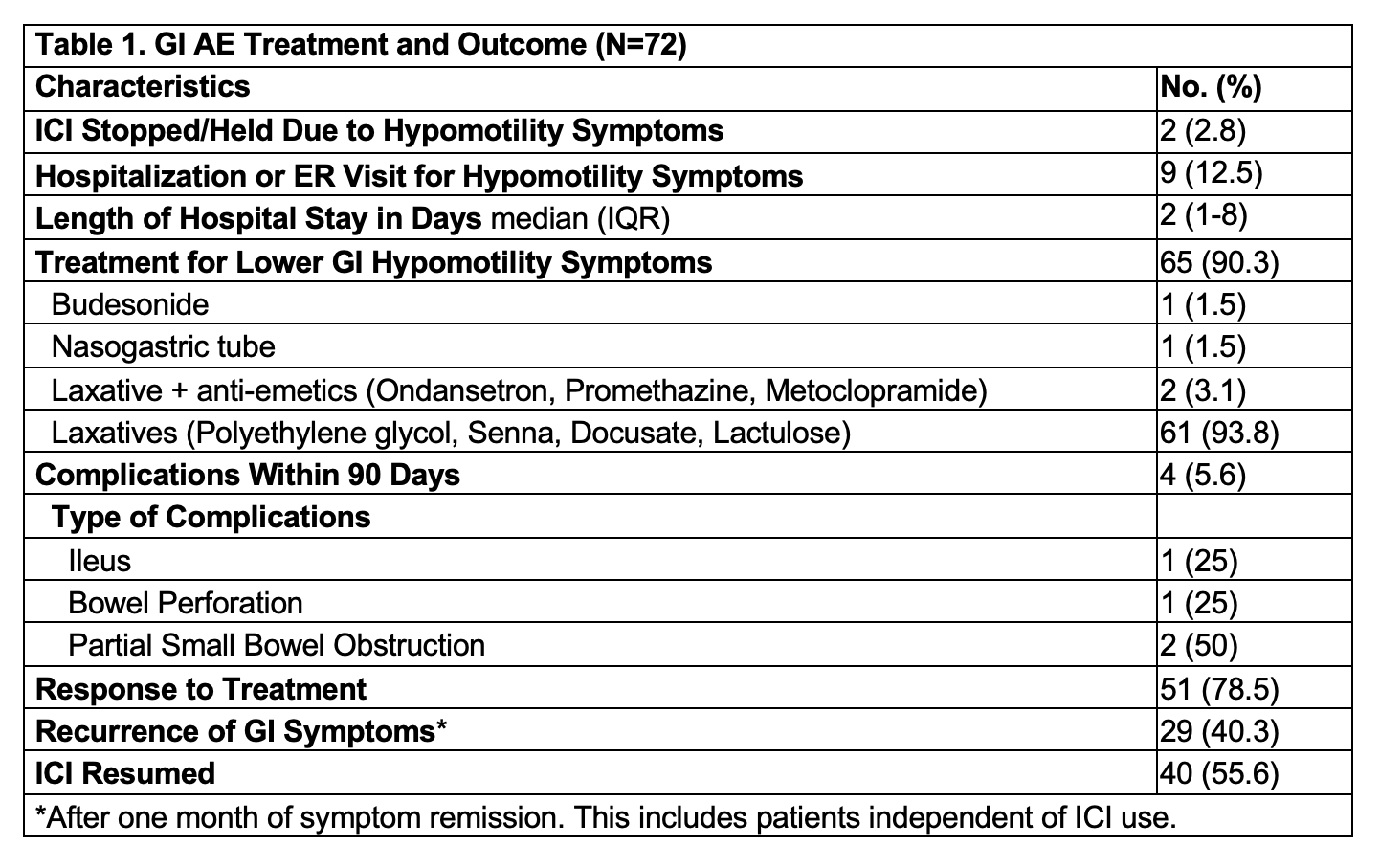
Figure: Table 1: GI Adverse Event Treatment and Outcome
Disclosures:
Raakhi Menon indicated no relevant financial relationships.
Sharada Wali indicated no relevant financial relationships.
Rohan Ahuja indicated no relevant financial relationships.
Samuel Black indicated no relevant financial relationships.
Anusha Shirwaikar Thomas indicated no relevant financial relationships.
Lavanya Viswanathan: Ardelyx, Inc. – Advisor or Review Panel Member, The above are unpaid., Speakers Bureau.
Mehnaz Shafi indicated no relevant financial relationships.
Yinghong Wang indicated no relevant financial relationships.
Raakhi Menon, DO1, Sharada Wali, MBBS, MPH2, Rohan Ahuja, MD3, Samuel Black, MD4, Anusha Shirwaikar Thomas, MD2, Lavanya Viswanathan, MD, MS, FACG5, Mehnaz Shafi, MD2, Yinghong Wang, MD, PhD, MS2. P0267 - Hypomotility of Lower Gastrointestinal Tract Post Immune Checkpoint Inhibitors, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1University of Texas Medical Branch, Galveston, TX; 2University of Texas MD Anderson Cancer Center, Houston, TX; 3University of Texas at Houston, Houston, TX; 4Baylor College of Medicine, Houston, TX; 5UT MD Anderson Cancer Center, Houston, TX
Introduction: Immune checkpoint inhibitors (ICIs) have transformed cancer therapy; however, their increased use has led to a rise in immune-related adverse events (irAEs). While diarrhea and colitis are well-documented gastrointestinal (GI) irAEs, emerging case studies now highlight ICI-related GI hypomotility. Constipation, though not typically classified as a GI irAE, is underreported in clinical practice, emphasizing the urgent need for further research.
Methods: This retrospective study analyzed adult patients with malignancies who developed constipation while receiving ICI therapy at a tertiary cancer center between 01/2010 and 02/2024 with deemed etiology related to ICI. Patients were excluded if symptoms were present prior to ICI therapy or attributed to other causes such as medications, comorbidities, surgeries, or mechanical obstructions. Clinical data and treatments as well as adverse events were reported.
Results: 72 patients were included, with a median age of 61 years (52-70), 59.7% male, and 70.8% Caucasian. Melanoma was the most common malignancy (34.7%), followed by hematological cancers (23.6%). Most patients (80.6%) received PD-1/L1 inhibitors. The median symptom duration was 20 days (8-48), with symptom onset occurring 96 days (34-172) post-ICI initiation. ICI was held in 2.8% of cases, and 12.5% required ER visits or hospitalization. Of note, 10 patients had both constipation and upper GI symptoms such as nausea/vomiting, with suspicion of a generalized motility disturbance. One patient underwent a colonoscopy, the pathology of which yielded non-specific findings.
Medical treatment for constipation was administered in 90.3% of cases, with complications occurring in 5.6%, including partial small bowel obstruction. Symptoms improved in 78.5% of patients, but 40.3% experienced recurrence within one month, and ICI was resumed in 55.6% of cases. Subgroup analysis showed that treatment responders (78.5%) had a median symptom duration of 24 days versus 105 days for non-responders. ICI was held in 2% of responders and 7.1% of non-responders. Recurrence rates after ICI resumption were much higher at 80.8% and 83.3%, respectively.
Discussion: While colitis is the most common gastrointestinal irAE, a subset of patients receiving ICI therapy may develop constipation. These findings suggest constipation may represent a less common but noteworthy adverse effect of ICI.

Figure: Figure 1. Inclusion and Exclusion Criteria

Figure: Table 1: GI Adverse Event Treatment and Outcome
Disclosures:
Raakhi Menon indicated no relevant financial relationships.
Sharada Wali indicated no relevant financial relationships.
Rohan Ahuja indicated no relevant financial relationships.
Samuel Black indicated no relevant financial relationships.
Anusha Shirwaikar Thomas indicated no relevant financial relationships.
Lavanya Viswanathan: Ardelyx, Inc. – Advisor or Review Panel Member, The above are unpaid., Speakers Bureau.
Mehnaz Shafi indicated no relevant financial relationships.
Yinghong Wang indicated no relevant financial relationships.
Raakhi Menon, DO1, Sharada Wali, MBBS, MPH2, Rohan Ahuja, MD3, Samuel Black, MD4, Anusha Shirwaikar Thomas, MD2, Lavanya Viswanathan, MD, MS, FACG5, Mehnaz Shafi, MD2, Yinghong Wang, MD, PhD, MS2. P0267 - Hypomotility of Lower Gastrointestinal Tract Post Immune Checkpoint Inhibitors, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
