Sunday Poster Session
Category: Biliary/Pancreas
P0074 - Feasibility of Biliary and Pancreatic Drainage With an EUS-Guided Cystotome
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall

Hadi Khaled Abou Zeid, MD
Mayo Clinic
Rochester, MN
Presenting Author(s)
Hadi Khaled. Abou Zeid, MD1, Yara Salameh, MD1, Rishad Khan, MD1, Barham Abu Dayyeh, MD, MPH2, Ryan Law, DO1, Andrew Storm, MD1
1Mayo Clinic, Rochester, MN; 2Cedars-Sinai Medical Center, Los Angeles, CA
Introduction: An endoscopic ultrasound (EUS)-guided Cystotome, recently approved by the FDA, is an electrosurgically enhanced puncture device designed for use in EUS-guided access procedures. This study aimed to evaluate the safety and efficacy of the EUS Cystotome in Endoscopic Ultrasound-guided Biliary and Pancreatic Drainage (EUS-BPD).
Methods: We conducted a retrospective analysis of 12 patients who underwent Cystotome-assisted EUS-BPD. After confirming an optimal access location with EUS, the cystotome was used to puncture the duct and create a tract through which an angled guidewire was inserted into the biliary or pancreatic tree to guide dilation and stenting as needed. Technical success was defined by successful duct access, while clinical success was assessed based on presenting symptom relief. Intra- and post-procedural complications were recorded, with follow-up for re-intervention, recurrence, and long-term complications.
Results: The procedure achieved technical and clinical success rates of 100 and 75%, respectively. Post-procedural adverse events were noted in 4 cases (33%), ranging from 2 mild (epigastric pain, pancreatitis) to 2 severe complications, including pancreatitis, pancreatic leak, bleeding, infected intra-abdominal fluid collections, and pleural effusions. Notably, these severe complications were observed in patients undergoing pancreatic duct access procedures in the setting of prior Whipple procedures and suspected pancreaticojejunostomy strictures. At a mean follow-up of 4.42 ± 2.38 months, 2 cases required re-intervention due to stent migration, and 1 case due to failure to access the pancreatic duct through the pancreaticogastrostomy. Patient characteristics and procedure outcomes and details are highlighted in Tables 1 and 2.
Discussion: The EUS-guided Cystotome appears to be an effective alternative to ERCP in Biliary and Pancreatic Drainage, offering high technical and clinical success rates. While most complications were mild, severe adverse events highlight the need for additional studies to assess outcomes and safety, particularly in highly technical and complex cases.
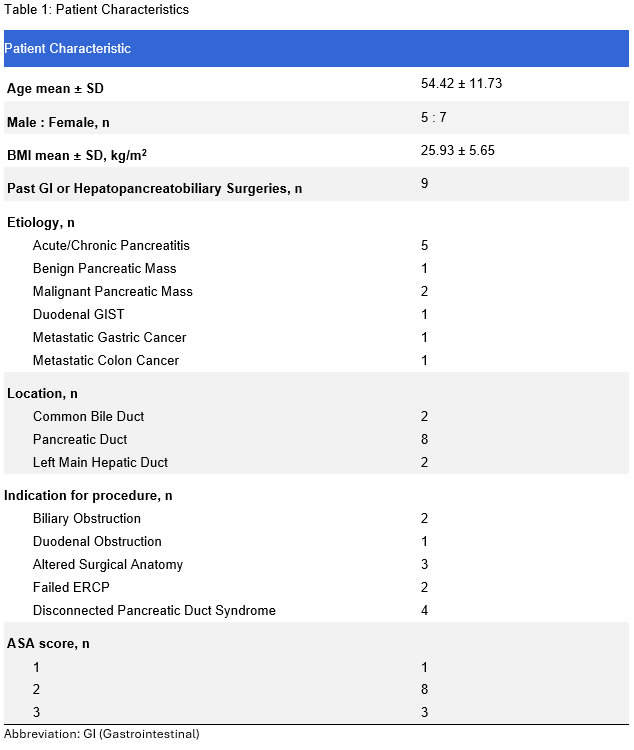
Figure: Table 1: Patient Characteristics
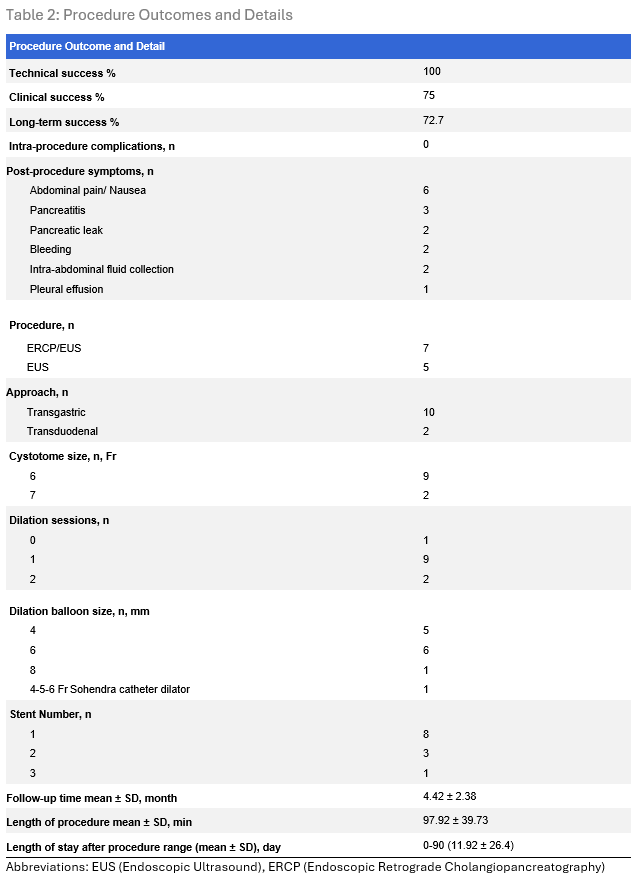
Figure: Table 2: Procedure Outcomes and Details
Disclosures:
Hadi Abou Zeid indicated no relevant financial relationships.
Yara Salameh indicated no relevant financial relationships.
Rishad Khan indicated no relevant financial relationships.
Barham Abu Dayyeh: Boston Scientific, Medtronic, Apollo Endosurgery, and Olympus – Consultant. Boston Scientific, Medtronic, Apollo Endosurgery, and USGI Medical – Grant/Research Support. Endogenex technology licensed by Mayo Clinic, with institutional equity and royalty through Mayo Clinic's invention policy. – coinventor.
Ryan Law: Boston Scientific – Consultant, Grant/Research Support. Neptune Medical – Data safety monitoring board. Olympus America – Consultant, Grant/Research Support. UpToDate – Royalties.
Andrew Storm: Ambu – Consultant. Apollo Endosurgery – Consultant, Grant/Research Support. Boston Scientific – Consultant, Grant/Research Support. Cook – Consultant. Endogenex – Grant/Research Support. Endo-Tagss – Grant/Research Support. Enterasense – Grant/Research Support. Envision Endoscopy – Grant/Research Support. Intuitive – Consultant. Medtronic – Consultant. MGI Medical – Grant/Research Support. Microtech – Consultant. Olympus – Consultant. OnePass – Grant/Research Support. SofTac – Grant/Research Support. Sotelix – Consultant. Steris – Consultant.
Hadi Khaled. Abou Zeid, MD1, Yara Salameh, MD1, Rishad Khan, MD1, Barham Abu Dayyeh, MD, MPH2, Ryan Law, DO1, Andrew Storm, MD1. P0074 - Feasibility of Biliary and Pancreatic Drainage With an EUS-Guided Cystotome, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Mayo Clinic, Rochester, MN; 2Cedars-Sinai Medical Center, Los Angeles, CA
Introduction: An endoscopic ultrasound (EUS)-guided Cystotome, recently approved by the FDA, is an electrosurgically enhanced puncture device designed for use in EUS-guided access procedures. This study aimed to evaluate the safety and efficacy of the EUS Cystotome in Endoscopic Ultrasound-guided Biliary and Pancreatic Drainage (EUS-BPD).
Methods: We conducted a retrospective analysis of 12 patients who underwent Cystotome-assisted EUS-BPD. After confirming an optimal access location with EUS, the cystotome was used to puncture the duct and create a tract through which an angled guidewire was inserted into the biliary or pancreatic tree to guide dilation and stenting as needed. Technical success was defined by successful duct access, while clinical success was assessed based on presenting symptom relief. Intra- and post-procedural complications were recorded, with follow-up for re-intervention, recurrence, and long-term complications.
Results: The procedure achieved technical and clinical success rates of 100 and 75%, respectively. Post-procedural adverse events were noted in 4 cases (33%), ranging from 2 mild (epigastric pain, pancreatitis) to 2 severe complications, including pancreatitis, pancreatic leak, bleeding, infected intra-abdominal fluid collections, and pleural effusions. Notably, these severe complications were observed in patients undergoing pancreatic duct access procedures in the setting of prior Whipple procedures and suspected pancreaticojejunostomy strictures. At a mean follow-up of 4.42 ± 2.38 months, 2 cases required re-intervention due to stent migration, and 1 case due to failure to access the pancreatic duct through the pancreaticogastrostomy. Patient characteristics and procedure outcomes and details are highlighted in Tables 1 and 2.
Discussion: The EUS-guided Cystotome appears to be an effective alternative to ERCP in Biliary and Pancreatic Drainage, offering high technical and clinical success rates. While most complications were mild, severe adverse events highlight the need for additional studies to assess outcomes and safety, particularly in highly technical and complex cases.

Figure: Table 1: Patient Characteristics

Figure: Table 2: Procedure Outcomes and Details
Disclosures:
Hadi Abou Zeid indicated no relevant financial relationships.
Yara Salameh indicated no relevant financial relationships.
Rishad Khan indicated no relevant financial relationships.
Barham Abu Dayyeh: Boston Scientific, Medtronic, Apollo Endosurgery, and Olympus – Consultant. Boston Scientific, Medtronic, Apollo Endosurgery, and USGI Medical – Grant/Research Support. Endogenex technology licensed by Mayo Clinic, with institutional equity and royalty through Mayo Clinic's invention policy. – coinventor.
Ryan Law: Boston Scientific – Consultant, Grant/Research Support. Neptune Medical – Data safety monitoring board. Olympus America – Consultant, Grant/Research Support. UpToDate – Royalties.
Andrew Storm: Ambu – Consultant. Apollo Endosurgery – Consultant, Grant/Research Support. Boston Scientific – Consultant, Grant/Research Support. Cook – Consultant. Endogenex – Grant/Research Support. Endo-Tagss – Grant/Research Support. Enterasense – Grant/Research Support. Envision Endoscopy – Grant/Research Support. Intuitive – Consultant. Medtronic – Consultant. MGI Medical – Grant/Research Support. Microtech – Consultant. Olympus – Consultant. OnePass – Grant/Research Support. SofTac – Grant/Research Support. Sotelix – Consultant. Steris – Consultant.
Hadi Khaled. Abou Zeid, MD1, Yara Salameh, MD1, Rishad Khan, MD1, Barham Abu Dayyeh, MD, MPH2, Ryan Law, DO1, Andrew Storm, MD1. P0074 - Feasibility of Biliary and Pancreatic Drainage With an EUS-Guided Cystotome, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
