Sunday Poster Session
Category: Biliary/Pancreas
P0073 - Assessing the Safety and Efficacy of a Novel EUS-Guided Cystotome in Drainage Procedures
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall

Hadi Khaled Abou Zeid, MD
Mayo Clinic
Rochester, MN
Presenting Author(s)
Hadi Khaled. Abou Zeid, MD1, Yara Salameh, MD1, Rishad Khan, MD1, Barham Abu Dayyeh, MD, MPH2, Ryan Law, DO1, Andrew Storm, MD1
1Mayo Clinic, Rochester, MN; 2Cedars-Sinai Medical Center, Los Angeles, CA
Introduction: The Cystotome is a novel Endoscopic Ultrasound (EUS)-guided electrosurgical accessory that facilitates EUS-guided transmural drainage of Pancreatic Fluid Collections (PFCs). This study aims to assess the safety and efficacy of cystotome-assisted PFC drainage.
Methods: A retrospective analysis was conducted. The procedure involved the identification of an appropriate location via Color Doppler to avoid interposed vessels, cyst puncture, and insertion of an angled wire. Subsequently, the EUS-guided cystotome was introduced to dilate the fistula where stents were placed. Technical success was defined as the successful insertion of the cystotome and the creation of an appropriate drainage tract. Clinical success was defined as the improvement in patients’ symptoms. Long-term outcomes included recurrence rates, the need for additional procedures, and long-term resolution of PFC complications.
Results: A total of 14 patients (mean age 56.8 ±15.6, 35.7% females) were included. The majority of PFCs were pseudocysts (71.4%) with an average size of 51.27 x 40.47 mm (± 27.95 x 21.37) (Table 1). The procedure had technical and clinical success rates of 100 and 83.3%, respectively. No intraprocedural complications were observed. The overall postprocedural complications were observed in 14.3% of patients, including 1 moderate complication (pseudocyst infection) and 1 severe complication (hypoxemic respiratory distress). Long-term success was achieved in 75% of patients with one case of recurrence and two cases requiring re-intervention (Table 2).
Discussion: The cystotome is a safe and effective tool for EUS-guided PFC drainage, demonstrating high technical and clinical success rates with an acceptable complication profile. Prospective studies are needed to further validate its role in complex cases.
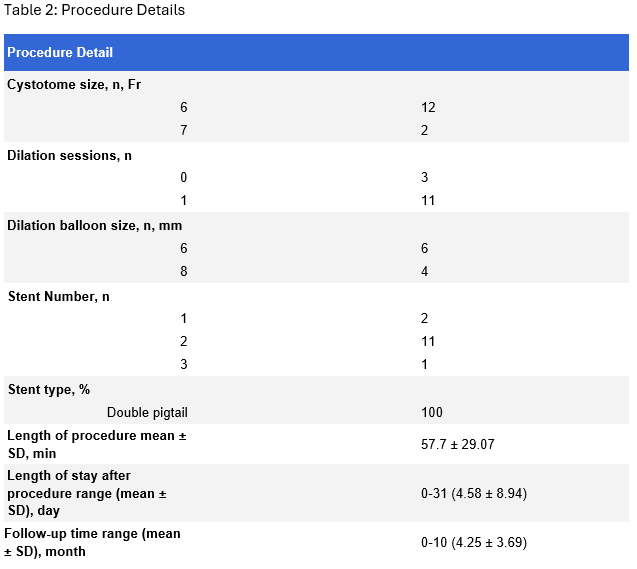
Figure: Table 1: Patient Characteristics
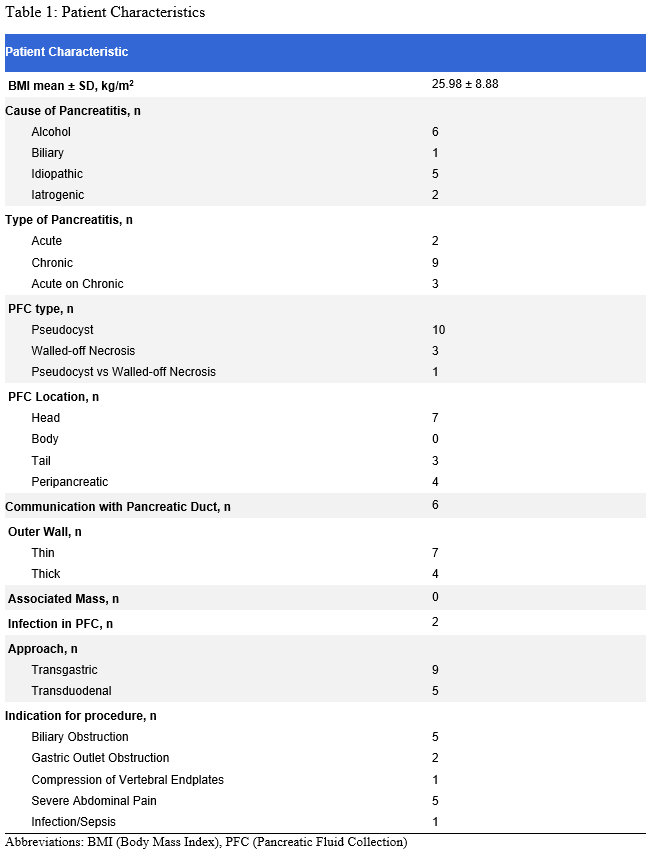
Figure: Table 2: Procedure Details
Disclosures:
Hadi Abou Zeid indicated no relevant financial relationships.
Yara Salameh indicated no relevant financial relationships.
Rishad Khan indicated no relevant financial relationships.
Barham Abu Dayyeh: Boston Scientific, Medtronic, Apollo Endosurgery, and Olympus – Consultant. Boston Scientific, Medtronic, Apollo Endosurgery, and USGI Medical – Grant/Research Support. Endogenex technology licensed by Mayo Clinic, with institutional equity and royalty through Mayo Clinic's invention policy. – coinventor.
Ryan Law: Boston Scientific – Consultant, Grant/Research Support. Neptune Medical – Data safety monitoring board. Olympus America – Consultant, Grant/Research Support. UpToDate – Royalties.
Andrew Storm: Ambu – Consultant. Apollo Endosurgery – Consultant, Grant/Research Support. Boston Scientific – Consultant, Grant/Research Support. Cook – Consultant. Endogenex – Grant/Research Support. Endo-Tagss – Grant/Research Support. Enterasense – Grant/Research Support. Envision Endoscopy – Grant/Research Support. Intuitive – Consultant. Medtronic – Consultant. MGI Medical – Grant/Research Support. Microtech – Consultant. Olympus – Consultant. OnePass – Grant/Research Support. SofTac – Grant/Research Support. Sotelix – Consultant. Steris – Consultant.
Hadi Khaled. Abou Zeid, MD1, Yara Salameh, MD1, Rishad Khan, MD1, Barham Abu Dayyeh, MD, MPH2, Ryan Law, DO1, Andrew Storm, MD1. P0073 - Assessing the Safety and Efficacy of a Novel EUS-Guided Cystotome in Drainage Procedures, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Mayo Clinic, Rochester, MN; 2Cedars-Sinai Medical Center, Los Angeles, CA
Introduction: The Cystotome is a novel Endoscopic Ultrasound (EUS)-guided electrosurgical accessory that facilitates EUS-guided transmural drainage of Pancreatic Fluid Collections (PFCs). This study aims to assess the safety and efficacy of cystotome-assisted PFC drainage.
Methods: A retrospective analysis was conducted. The procedure involved the identification of an appropriate location via Color Doppler to avoid interposed vessels, cyst puncture, and insertion of an angled wire. Subsequently, the EUS-guided cystotome was introduced to dilate the fistula where stents were placed. Technical success was defined as the successful insertion of the cystotome and the creation of an appropriate drainage tract. Clinical success was defined as the improvement in patients’ symptoms. Long-term outcomes included recurrence rates, the need for additional procedures, and long-term resolution of PFC complications.
Results: A total of 14 patients (mean age 56.8 ±15.6, 35.7% females) were included. The majority of PFCs were pseudocysts (71.4%) with an average size of 51.27 x 40.47 mm (± 27.95 x 21.37) (Table 1). The procedure had technical and clinical success rates of 100 and 83.3%, respectively. No intraprocedural complications were observed. The overall postprocedural complications were observed in 14.3% of patients, including 1 moderate complication (pseudocyst infection) and 1 severe complication (hypoxemic respiratory distress). Long-term success was achieved in 75% of patients with one case of recurrence and two cases requiring re-intervention (Table 2).
Discussion: The cystotome is a safe and effective tool for EUS-guided PFC drainage, demonstrating high technical and clinical success rates with an acceptable complication profile. Prospective studies are needed to further validate its role in complex cases.

Figure: Table 1: Patient Characteristics

Figure: Table 2: Procedure Details
Disclosures:
Hadi Abou Zeid indicated no relevant financial relationships.
Yara Salameh indicated no relevant financial relationships.
Rishad Khan indicated no relevant financial relationships.
Barham Abu Dayyeh: Boston Scientific, Medtronic, Apollo Endosurgery, and Olympus – Consultant. Boston Scientific, Medtronic, Apollo Endosurgery, and USGI Medical – Grant/Research Support. Endogenex technology licensed by Mayo Clinic, with institutional equity and royalty through Mayo Clinic's invention policy. – coinventor.
Ryan Law: Boston Scientific – Consultant, Grant/Research Support. Neptune Medical – Data safety monitoring board. Olympus America – Consultant, Grant/Research Support. UpToDate – Royalties.
Andrew Storm: Ambu – Consultant. Apollo Endosurgery – Consultant, Grant/Research Support. Boston Scientific – Consultant, Grant/Research Support. Cook – Consultant. Endogenex – Grant/Research Support. Endo-Tagss – Grant/Research Support. Enterasense – Grant/Research Support. Envision Endoscopy – Grant/Research Support. Intuitive – Consultant. Medtronic – Consultant. MGI Medical – Grant/Research Support. Microtech – Consultant. Olympus – Consultant. OnePass – Grant/Research Support. SofTac – Grant/Research Support. Sotelix – Consultant. Steris – Consultant.
Hadi Khaled. Abou Zeid, MD1, Yara Salameh, MD1, Rishad Khan, MD1, Barham Abu Dayyeh, MD, MPH2, Ryan Law, DO1, Andrew Storm, MD1. P0073 - Assessing the Safety and Efficacy of a Novel EUS-Guided Cystotome in Drainage Procedures, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
