Sunday Poster Session
Category: Biliary/Pancreas
P0065 - Revolutionizing Biliary Diagnostics: The Role of AI in Enhancing Choledocholithiasis Diagnosis -- A Systematic Review and Meta-Analysis
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall

Panagiotis G. Doukas, MD (he/him/his)
Saint Peter's University Hospital / Rutgers Robert Wood Johnson Medical School
New Brunswick, NJ
Presenting Author(s)
Panagiotis G.. Doukas, MD, Sotirios G.. Doukas, MD, PhD, Arkady Broder, MD, FACG
Saint Peter's University Hospital / Rutgers Robert Wood Johnson Medical School, New Brunswick, NJ
Introduction: Accurate diagnosis of choledocholithiasis (CDL) is essential to avoid delayed treatment, prevent complications, and reduce unnecessary interventions. Traditional guidelines, such as those from the American Society for Gastrointestinal Endoscopy (ASGE), aid in risk stratification but may lack precision. Artificial intelligence (AI) and machine learning (ML) offer emerging diagnostic tools that may enhance the accuracy and timeliness of CDL prediction. We aimed to systematically evaluate the diagnostic performance of AI-assisted tools in predicting CDL and compare it to traditional guideline-based methods
Methods: A comprehensive search was conducted in MEDLINE, EMBASE, PubMed, and Web of Science, identifying 578 studies. After screening and application of inclusion criteria, 11 studies were analyzed. A bivariate random-effects model was used to pool sensitivity, specificity, and positive likelihood ratios (LR+). Summary receiver operating characteristics (SROC) curves were also generated.
Results: Meta-analysis showed an overall high pool sensitivity and specificity of AI-assisted models were 83.2% [95% confidence interval (CI): 68.9; 91.8] and 91.1% [95% CI: 84.7; 95], respectively (Figure 1A, 1B). The LR+ from the common effect model was 8.39 [7.41; 9.50], suggesting that AI models have a moderately strong ability to predict CDL. AI models demonstrated higher diagnostic performance than traditional ASGE guidelines, as evidenced by SROC comparisons (Figure 2A, 2B).
Discussion: AI-assisted tools show promise in enhancing CDL diagnosis through high sensitivity and specificity. While preliminary results are encouraging, further prospective studies are necessary to validate these tools and facilitate integration into routine clinical practice.
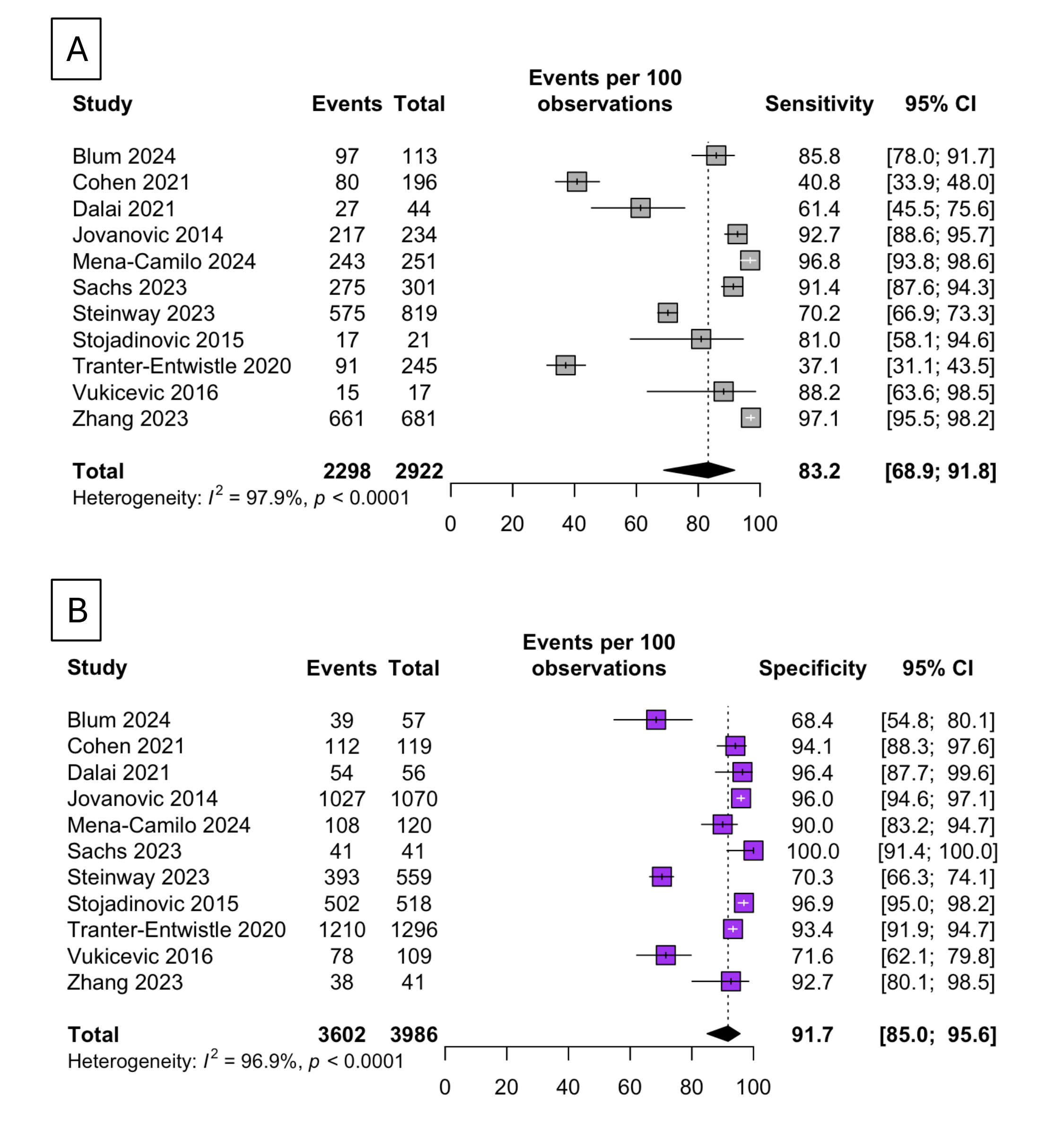
Figure: Figure 1: A. Forrest plot displaying pooled sensitivity estimates across all studies. B. Forrest plot displaying pooled specificity estimates across included studies, highlighting consistently strong diagnostic accuracy among diverse machine learning models.
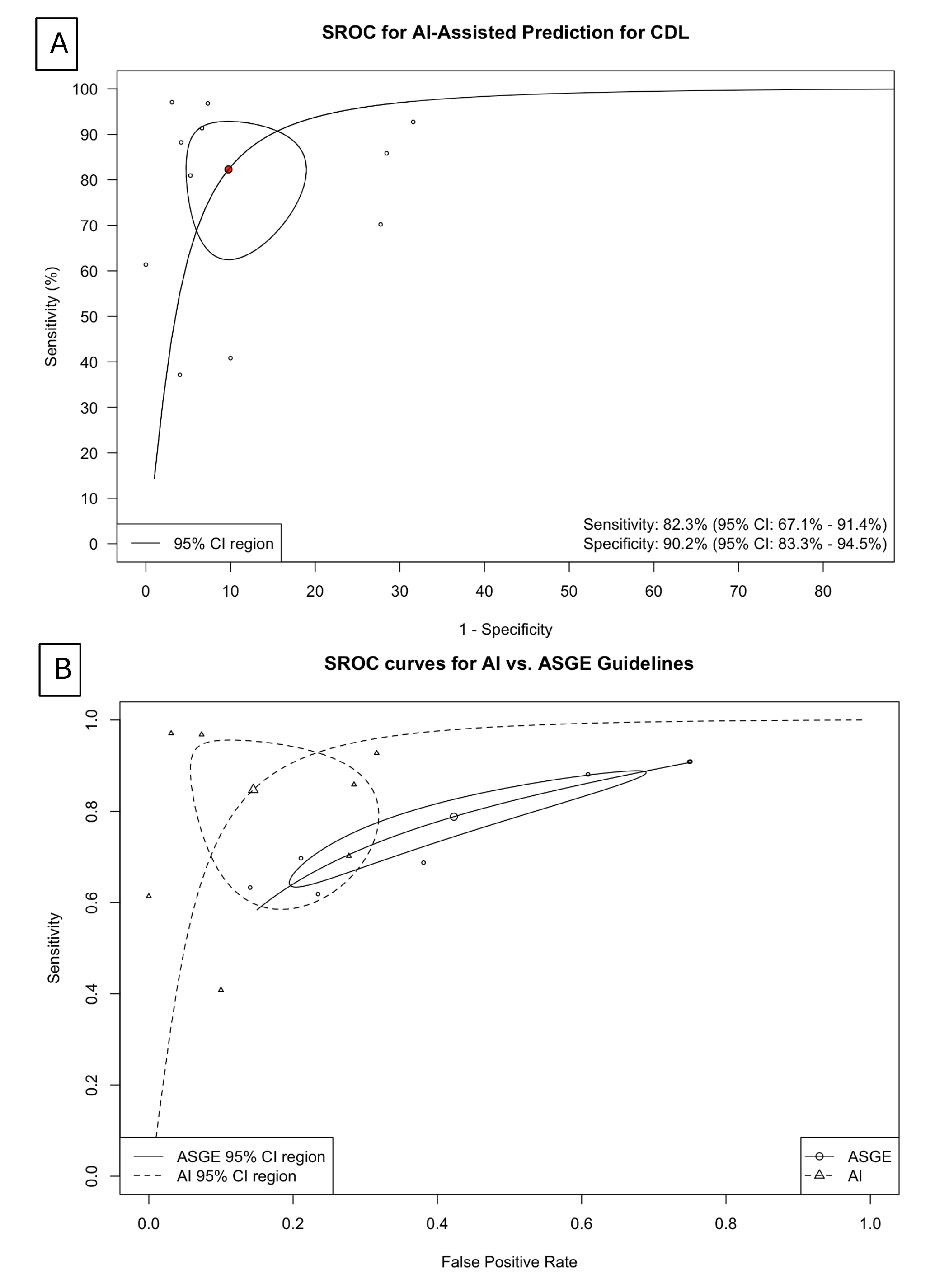
Figure: Figure 2: A. Bivariant analysis of SROC for AI-assisted prediction of choledocholithiasis. The SROC curve illustrates the overall diagnostic performance of AI-assisted tools. The pooled area under the curve (AUC) demonstrates high discriminative ability, supporting the robustness of AI models across diverse clinical populations. B. Comparative SROC Curves: AI Models vs. 2019 ASGE Guidelines, subgroup analysis. AUC for the AI group was 0.91 with 97.5% CI [0.76-0.96] and 0.72 with 97.5% CI [0.72-0.79]. AI models demonstrate a higher pooled AUC compared to ASGE criteria, suggesting improved accuracy for CDL prediction.
Disclosures:
Panagiotis Doukas indicated no relevant financial relationships.
Sotirios Doukas indicated no relevant financial relationships.
Arkady Broder indicated no relevant financial relationships.
Panagiotis G.. Doukas, MD, Sotirios G.. Doukas, MD, PhD, Arkady Broder, MD, FACG. P0065 - Revolutionizing Biliary Diagnostics: The Role of AI in Enhancing Choledocholithiasis Diagnosis -- A Systematic Review and Meta-Analysis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
Saint Peter's University Hospital / Rutgers Robert Wood Johnson Medical School, New Brunswick, NJ
Introduction: Accurate diagnosis of choledocholithiasis (CDL) is essential to avoid delayed treatment, prevent complications, and reduce unnecessary interventions. Traditional guidelines, such as those from the American Society for Gastrointestinal Endoscopy (ASGE), aid in risk stratification but may lack precision. Artificial intelligence (AI) and machine learning (ML) offer emerging diagnostic tools that may enhance the accuracy and timeliness of CDL prediction. We aimed to systematically evaluate the diagnostic performance of AI-assisted tools in predicting CDL and compare it to traditional guideline-based methods
Methods: A comprehensive search was conducted in MEDLINE, EMBASE, PubMed, and Web of Science, identifying 578 studies. After screening and application of inclusion criteria, 11 studies were analyzed. A bivariate random-effects model was used to pool sensitivity, specificity, and positive likelihood ratios (LR+). Summary receiver operating characteristics (SROC) curves were also generated.
Results: Meta-analysis showed an overall high pool sensitivity and specificity of AI-assisted models were 83.2% [95% confidence interval (CI): 68.9; 91.8] and 91.1% [95% CI: 84.7; 95], respectively (Figure 1A, 1B). The LR+ from the common effect model was 8.39 [7.41; 9.50], suggesting that AI models have a moderately strong ability to predict CDL. AI models demonstrated higher diagnostic performance than traditional ASGE guidelines, as evidenced by SROC comparisons (Figure 2A, 2B).
Discussion: AI-assisted tools show promise in enhancing CDL diagnosis through high sensitivity and specificity. While preliminary results are encouraging, further prospective studies are necessary to validate these tools and facilitate integration into routine clinical practice.

Figure: Figure 1: A. Forrest plot displaying pooled sensitivity estimates across all studies. B. Forrest plot displaying pooled specificity estimates across included studies, highlighting consistently strong diagnostic accuracy among diverse machine learning models.

Figure: Figure 2: A. Bivariant analysis of SROC for AI-assisted prediction of choledocholithiasis. The SROC curve illustrates the overall diagnostic performance of AI-assisted tools. The pooled area under the curve (AUC) demonstrates high discriminative ability, supporting the robustness of AI models across diverse clinical populations. B. Comparative SROC Curves: AI Models vs. 2019 ASGE Guidelines, subgroup analysis. AUC for the AI group was 0.91 with 97.5% CI [0.76-0.96] and 0.72 with 97.5% CI [0.72-0.79]. AI models demonstrate a higher pooled AUC compared to ASGE criteria, suggesting improved accuracy for CDL prediction.
Disclosures:
Panagiotis Doukas indicated no relevant financial relationships.
Sotirios Doukas indicated no relevant financial relationships.
Arkady Broder indicated no relevant financial relationships.
Panagiotis G.. Doukas, MD, Sotirios G.. Doukas, MD, PhD, Arkady Broder, MD, FACG. P0065 - Revolutionizing Biliary Diagnostics: The Role of AI in Enhancing Choledocholithiasis Diagnosis -- A Systematic Review and Meta-Analysis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
