Oral Paper Presentation
Annual Scientific Meeting
Session: Plenary Session 1A: Infection, Microbiome, Small Intestine
11 - Significant and Durable Microbiome Restoration in a Phase 3 Trial of Fecal Microbiota, Live-JSLM for Recurrent Clostridioides difficile Infection When Administered by Colonoscopy
Monday, October 27, 2025
2:15 PM - 2:25 PM PDT
Location: North Ballroom 120D

Sahil Khanna, MBBS, MD, MS (he/him/his)
Mayo Clinic Rochester
Rochester, MN
Presenting Author(s)
Sahil Khanna, MBBS, MD, MS1, Paul Feuerstadt, MD, FACG2, Daniel Van Handel, MD3, Ken Blount, PhD4, Tonya Ward, PhD4, Beth Adamowicz, PhD4
1Mayo Clinic Rochester, Rochester, MN; 2Yale University School of Medicine and PACT-Gastroenterology Center, Hamden, CT; 3Plymouth Endoscopy Center & Clinic, Plymouth, MN; 4Ferring Pharmaceuticals, Roseville, MN
Introduction: Microbiota-based products may shift the microbial composition of a recipient’s gut microbiome to suppress Clostridioides difficile outgrowth and prevent recurrent C. difficile infection (rCDI). Fecal microbiota, live-jslm (RBL) is the first FDA-approved, single-dose, microbiota-based product indicated for the prevention of rCDI in adults after standard-of-care antibiotic therapy. The safety and clinical effectiveness of RBL when administered by colonoscopy to adults with rCDI was evaluated in CDI-SCOPE (NCT05831189), a phase 3b multi-center, single-arm trial. Microbiome composition, diversity of bacterial populations, and the Microbiome Health Index for post-Antibiotic dysbiosis (MHI-A) were assessed among RBL recipients in CDI-SCOPE.
Methods: These exploratory analyses included samples from 41 participants who received RBL in CDI-SCOPE. Treatment success was defined as the absence of Clostridioides difficile infection (CDI) diarrhea for 8 weeks after RBL administration. Participants were required to provide stool samples prior to bowel preparation and RBL administration by colonoscopy (baseline), at regular follow-up visits after 1, 4, and 8 weeks, after 3 and 6 months, and at the time of CDI recurrence within 8 weeks of RBL administration. Samples were extracted and sequenced using deep ( >20 MM reads) shotgun methods. Class-level relative abundances and alpha diversity were evaluated, including the MHI-A — a microbiome biomarker of post-antibiotic dysbiosis and restoration.
Results: Overall, 41 received RBL via colonoscopy; 39 participants completed an 8-week visit, with a treatment success rate of 95%.There were 20 participants with a full stool sample timeline (baseline, 1 week, 4 weeks, 8 weeks, 3 months, and 6 months) and 18 with a baseline sample and at least 1 post-administration sample. Compared with baseline, microbiome composition and MHI-A shifted significantly towards RBL composition among responders. Bacteroidia and Clostridia increased in abundance, whereas Gammaproteobacteria and Bacilli decreased following RBL administration via colonoscopy (Figure 1). MHI-A also increased from baseline to 6 months after RBL administration (Figure 2).
Discussion: RBL administered via colonoscopy restored microbiome composition concurrent with clinical response. These restorative changes reflect shifts from a post-antibiotic state of dysbiosis to a healthier state characterized by increased colonization resistance.
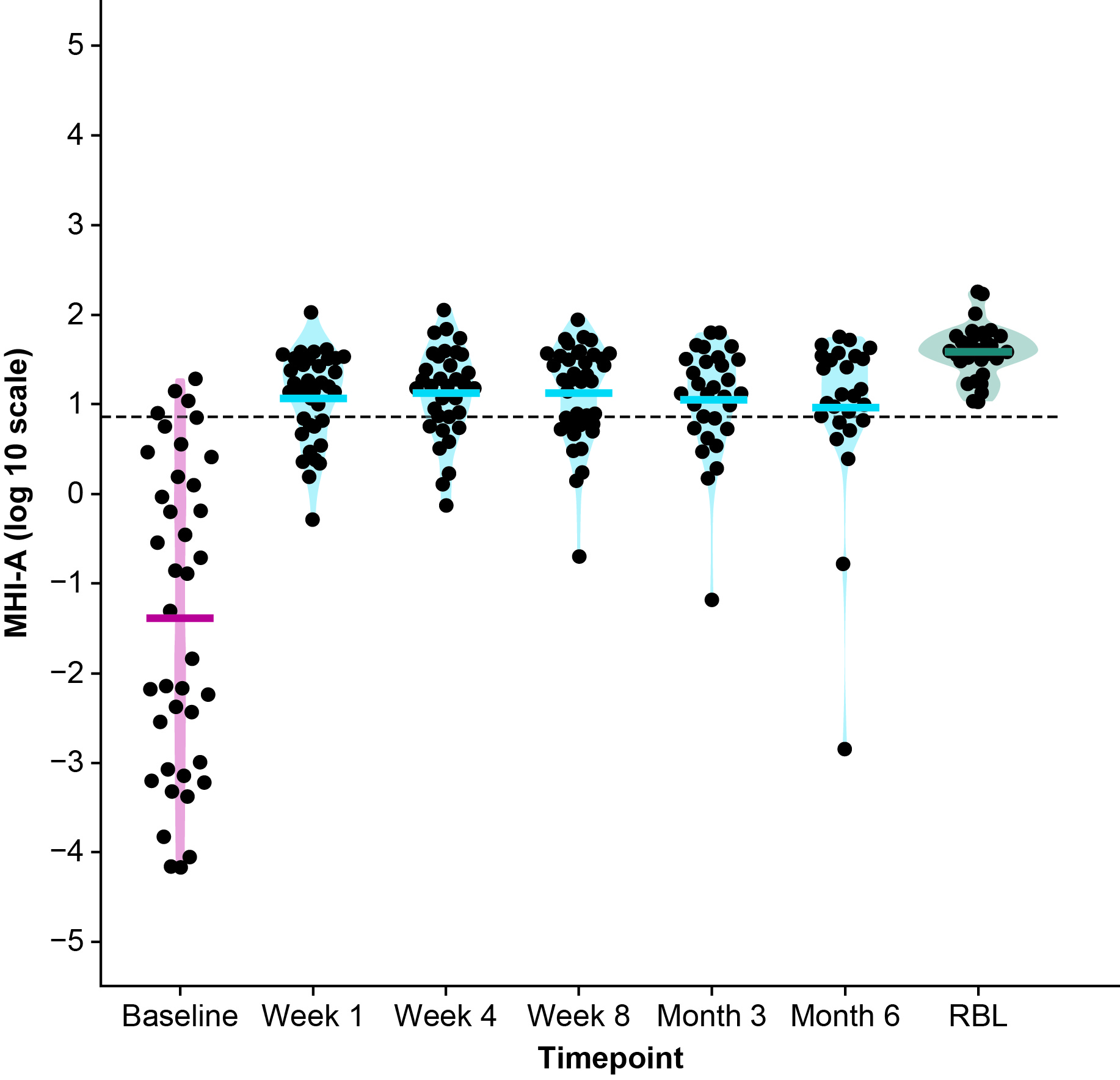
Figure: Figure 1. Relative abundance of key genera over time in treatment responders in CDI-SCOPE.
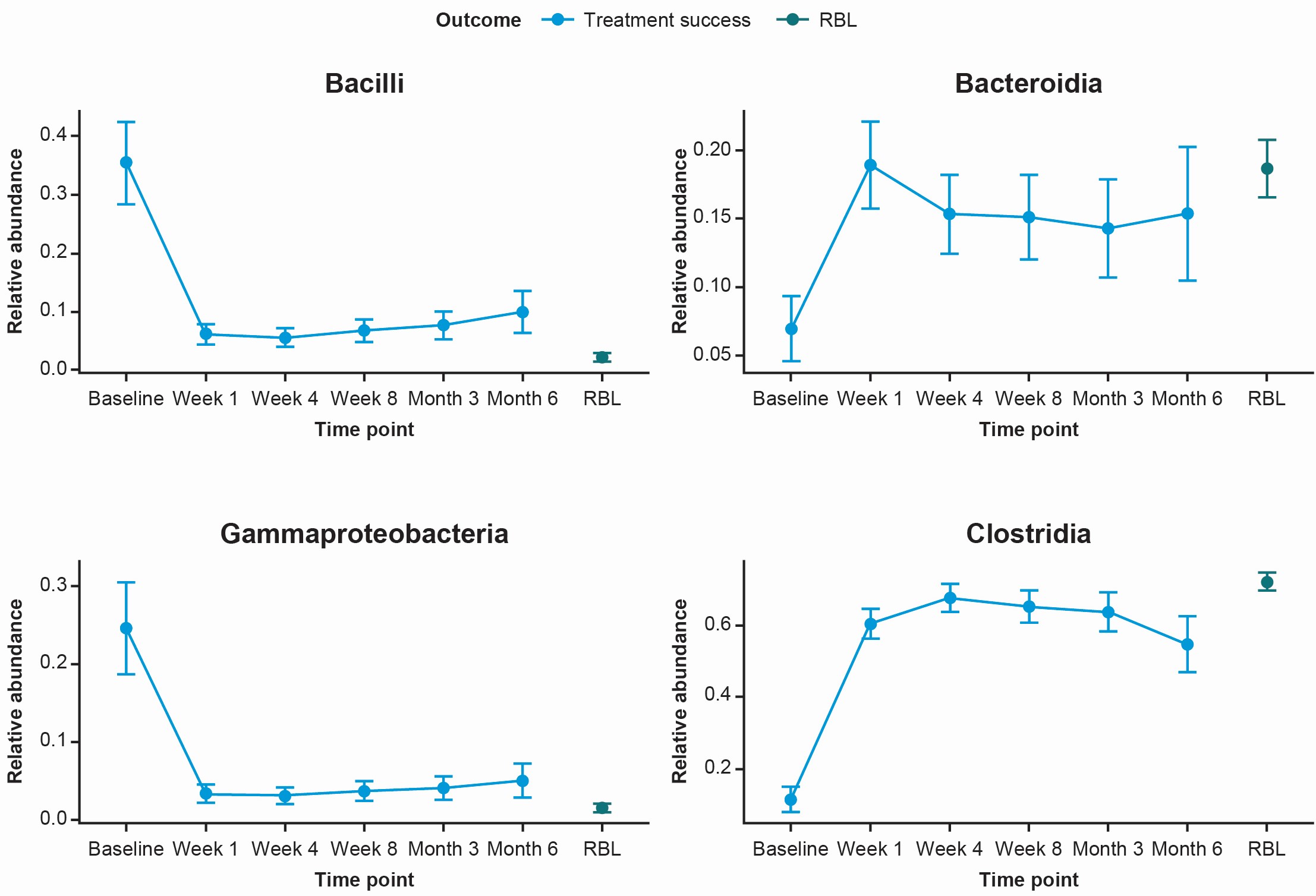
Figure: Figure 2. Microbiome Health Index for post-Antibiotic dysbiosis (MHI-A) values over time in treatment responders in CDI-SCOPE.
Disclosures:
Sahil Khanna: Ferring Pharmaceuticals – Advisor or Review Panel Member. Finch Therapeutics – Grant/Research Support. Immuron Limited – Consultant. Jetson Probiotics – Stock Options. Niche Pharmaceuticals – Consultant. Rebiotix – Grant/Research Support. Seres Therapeutics – Grant/Research Support. Vedanta BioSciences – Grant/Research Support.
Paul Feuerstadt: Ferring Pharmaceuticals – Advisor or Review Panel Member, Honoraria. Rebiotix, Inc. – Consultant. Seres Therapeutics – Advisor or Review Panel Member, Honoraria. Takeda Pharmaceuticals – Advisor or Review Panel Member, Consultant, Honoraria.
Daniel Van Handel: Ferring Pharmaceuticals – Speakers Bureau.
Ken Blount: Ferring Microbiome, Inc. – Employee.
Tonya Ward: Ferring Microbiome, Inc. – Employee.
Beth Adamowicz: Ferring Microbiome, Inc. – Speakers Bureau.
Sahil Khanna, MBBS, MD, MS1, Paul Feuerstadt, MD, FACG2, Daniel Van Handel, MD3, Ken Blount, PhD4, Tonya Ward, PhD4, Beth Adamowicz, PhD4, 11, Significant and Durable Microbiome Restoration in a Phase 3 Trial of Fecal Microbiota, Live-JSLM for Recurrent Clostridioides difficile Infection When Administered by Colonoscopy, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Mayo Clinic Rochester, Rochester, MN; 2Yale University School of Medicine and PACT-Gastroenterology Center, Hamden, CT; 3Plymouth Endoscopy Center & Clinic, Plymouth, MN; 4Ferring Pharmaceuticals, Roseville, MN
Introduction: Microbiota-based products may shift the microbial composition of a recipient’s gut microbiome to suppress Clostridioides difficile outgrowth and prevent recurrent C. difficile infection (rCDI). Fecal microbiota, live-jslm (RBL) is the first FDA-approved, single-dose, microbiota-based product indicated for the prevention of rCDI in adults after standard-of-care antibiotic therapy. The safety and clinical effectiveness of RBL when administered by colonoscopy to adults with rCDI was evaluated in CDI-SCOPE (NCT05831189), a phase 3b multi-center, single-arm trial. Microbiome composition, diversity of bacterial populations, and the Microbiome Health Index for post-Antibiotic dysbiosis (MHI-A) were assessed among RBL recipients in CDI-SCOPE.
Methods: These exploratory analyses included samples from 41 participants who received RBL in CDI-SCOPE. Treatment success was defined as the absence of Clostridioides difficile infection (CDI) diarrhea for 8 weeks after RBL administration. Participants were required to provide stool samples prior to bowel preparation and RBL administration by colonoscopy (baseline), at regular follow-up visits after 1, 4, and 8 weeks, after 3 and 6 months, and at the time of CDI recurrence within 8 weeks of RBL administration. Samples were extracted and sequenced using deep ( >20 MM reads) shotgun methods. Class-level relative abundances and alpha diversity were evaluated, including the MHI-A — a microbiome biomarker of post-antibiotic dysbiosis and restoration.
Results: Overall, 41 received RBL via colonoscopy; 39 participants completed an 8-week visit, with a treatment success rate of 95%.There were 20 participants with a full stool sample timeline (baseline, 1 week, 4 weeks, 8 weeks, 3 months, and 6 months) and 18 with a baseline sample and at least 1 post-administration sample. Compared with baseline, microbiome composition and MHI-A shifted significantly towards RBL composition among responders. Bacteroidia and Clostridia increased in abundance, whereas Gammaproteobacteria and Bacilli decreased following RBL administration via colonoscopy (Figure 1). MHI-A also increased from baseline to 6 months after RBL administration (Figure 2).
Discussion: RBL administered via colonoscopy restored microbiome composition concurrent with clinical response. These restorative changes reflect shifts from a post-antibiotic state of dysbiosis to a healthier state characterized by increased colonization resistance.

Figure: Figure 1. Relative abundance of key genera over time in treatment responders in CDI-SCOPE.

Figure: Figure 2. Microbiome Health Index for post-Antibiotic dysbiosis (MHI-A) values over time in treatment responders in CDI-SCOPE.
Disclosures:
Sahil Khanna: Ferring Pharmaceuticals – Advisor or Review Panel Member. Finch Therapeutics – Grant/Research Support. Immuron Limited – Consultant. Jetson Probiotics – Stock Options. Niche Pharmaceuticals – Consultant. Rebiotix – Grant/Research Support. Seres Therapeutics – Grant/Research Support. Vedanta BioSciences – Grant/Research Support.
Paul Feuerstadt: Ferring Pharmaceuticals – Advisor or Review Panel Member, Honoraria. Rebiotix, Inc. – Consultant. Seres Therapeutics – Advisor or Review Panel Member, Honoraria. Takeda Pharmaceuticals – Advisor or Review Panel Member, Consultant, Honoraria.
Daniel Van Handel: Ferring Pharmaceuticals – Speakers Bureau.
Ken Blount: Ferring Microbiome, Inc. – Employee.
Tonya Ward: Ferring Microbiome, Inc. – Employee.
Beth Adamowicz: Ferring Microbiome, Inc. – Speakers Bureau.
Sahil Khanna, MBBS, MD, MS1, Paul Feuerstadt, MD, FACG2, Daniel Van Handel, MD3, Ken Blount, PhD4, Tonya Ward, PhD4, Beth Adamowicz, PhD4, 11, Significant and Durable Microbiome Restoration in a Phase 3 Trial of Fecal Microbiota, Live-JSLM for Recurrent Clostridioides difficile Infection When Administered by Colonoscopy, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.


