Oral Paper Presentation
Annual Scientific Meeting
Session: Presidential Plenary Session 2
9 - Yield of Post-Polypectomy Interval Fecal Immunochemical Testing: Results From a Large Nationwide Veterans’ Affairs Database
Monday, October 27, 2025
10:06 AM - 10:18 AM PDT
Location: North Ballroom 120D

Natalie J. Wilson, MD (she/her/hers)
University of Minnesota
Chapel Hill, NC
Presenting Author(s)
Natalie J. Wilson, MD1, Mohammad Bilal, MD, FACG2, Anders Westanmo, PharmD, MBA3, Amy Gravely, MA4, Khalid Ishani, BS5, Rahul Karna, MD6, Aasma Shaukat, MD, MPH, FACG7
1University of Minnesota, Minneapolis, MN; 2University of Colorado Anschutz Medical Campus, Denver, CO; 3Minneapolis VA Health Care System, Minneapolis, MN; 4Minneapolis VA Medical Center, Research Service, Minneapolis, MN; 5University of Minnesota Medical School, Minneapolis, MN; 6University of Minnesota Medical Center, Minneapolis, MN; 7NYU Grossman School of Medicine, Division of Gastroenterology and Hepatology, New York, NY
Introduction: Current guidelines recommend surveillance colonoscopy after polypectomy. In real-world practice, fecal immunochemical testing (FIT) is sometimes used for surveillance after polypectomy, though little data exists to inform its use in this setting.
This study aims to evaluate the yield of interval FIT (iFIT) in patients with prior polypectomy compared to those without polypectomy, focusing on 1) the impact of polypectomy on iFIT results, 2) adherence to follow-up colonoscopy after a positive iFIT, and 3) to determine whether polypectomy is a predictor of advanced colorectal neoplasia (ACRN) or CRC after positive iFIT.
Methods: We analyzed data from the Veterans Health Administration Corporate Data Warehouse from 2000-2024. Patients who underwent colonoscopy for any indication followed by iFIT within 10 years and before another colonoscopy were included. Advanced colorectal neoplasia (ACRN) was defined as adenoma with high-grade dysplasia (HGD), tubulovillous (TVA), or CRC.
Results: Overall, 4,799,644 individuals underwent colonoscopy and 10.9% (525,157) had iFIT. Of these, 49.9% underwent polypectomy at index colonoscopy. Demographics and index colonoscopy findings are shown in Table 1.
The average time from index colonoscopy to iFIT was 5.9 years. iFIT was positive in 17.2% (44,931) of post-polypectomy patients vs 14.1% (37,081) without polypectomy. History of polypectomy was an independent predictor of positive iFIT (OR 1.12; 95% CI, 1.10–1.15; p< 0.0001). Follow-up colonoscopy after positive iFIT was performed in 50.4% of post-polypectomy patients and 49.3% without polypectomy. Findings on follow-up colonoscopy in those with and without polypectomy were as follows: tubular adenoma (54.7% vs 45.8%), TVA (5.6% vs 4.7%), adenoma with HGD (0.82% vs 0.66%), sessile serrated lesion (3.52% vs 2.42%), ACRN (9.2% vs 7.9%), and CRC (3.3% vs 3.0%), respectively (Table 2). Prior polypectomy was not an independent predictor of CRC (OR 0.96; 95% CI, 0.82–1.13; p=0.65) or ACRN (OR 0.97; 95% CI, 0.88–1.07; p=0.57) at colonoscopy post-iFIT.
Discussion: In this large US cohort, iFIT was performed frequently in patients with and without polypectomy at index colonoscopy, and was positive in 14-17%. Colonoscopy after positive iFIT revealed high rates of ACRN and CRC, irrespective of polypectomy history. These findings reinforce the importance of colonoscopy following positive iFIT given the high risk of ACRN and CRC, regardless of polypectomy history.
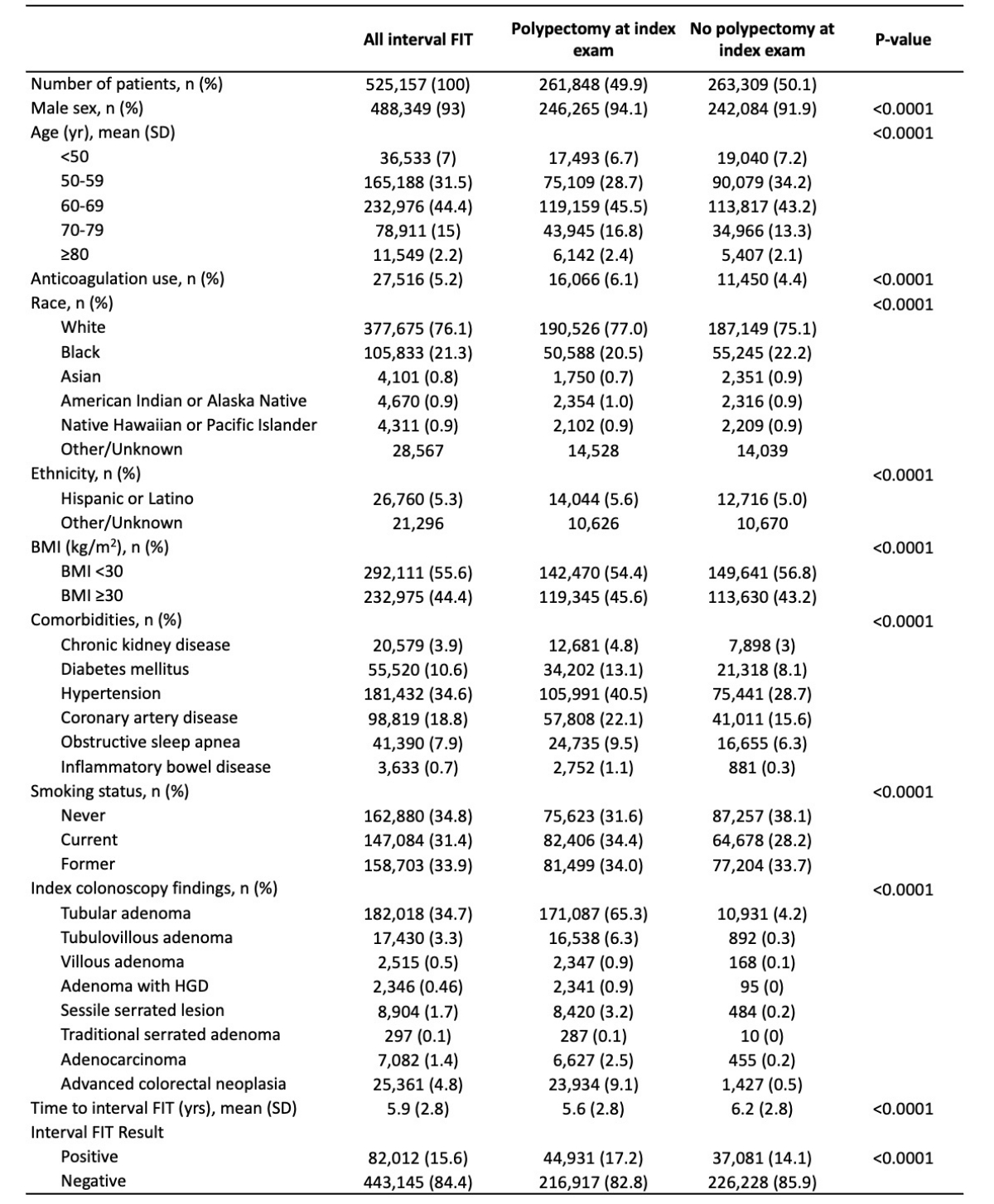
Figure: Table 1. Demographics and index colonoscopy findings, stratified by polypectomy history at index colonoscopy
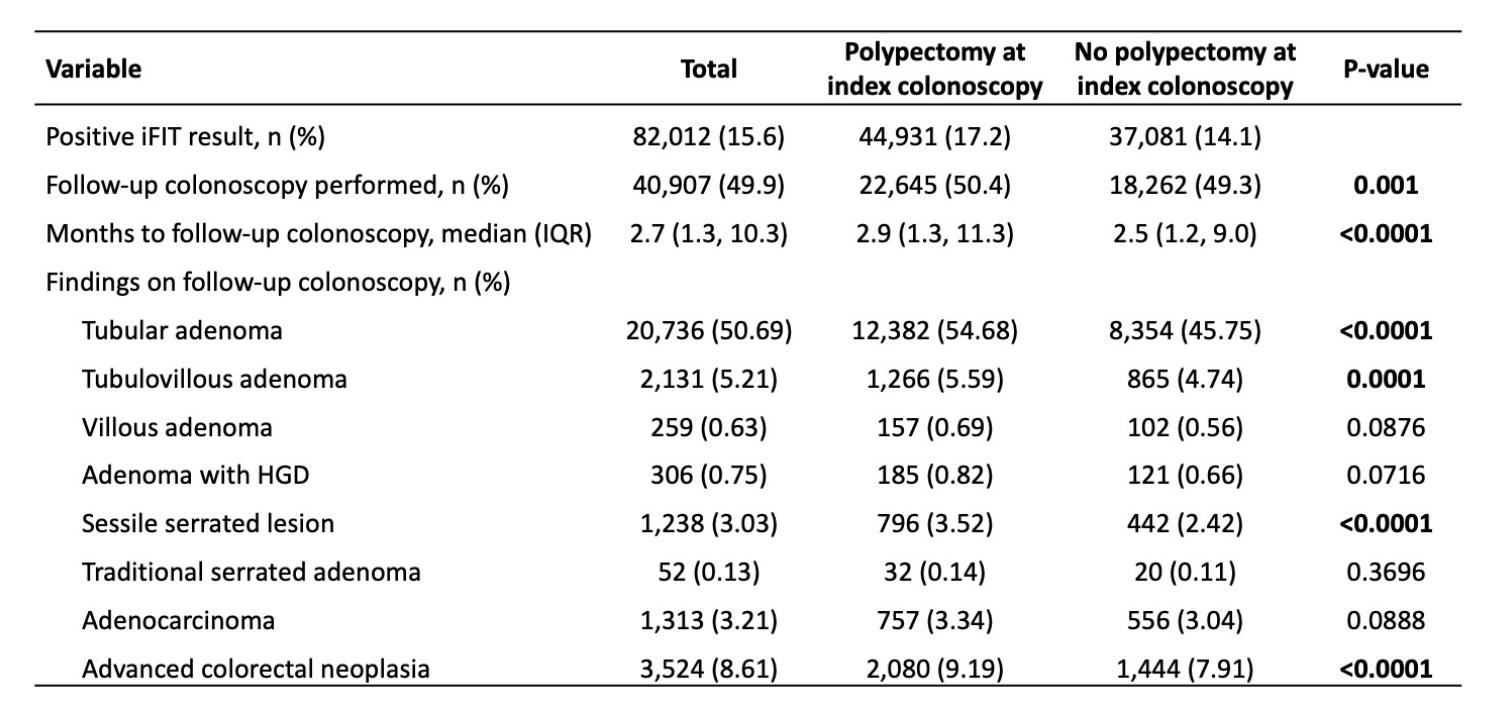
Figure: Table 2. Findings on colonoscopy performed after a positive interval FIT, stratified by history of polypectomy at index colonoscopy
Disclosures:
Natalie Wilson indicated no relevant financial relationships.
Mohammad Bilal: Boston Scientific – Consultant. Cook endoscopy – Paid speaker. Steris Endoscopy – Consultant.
Anders Westanmo indicated no relevant financial relationships.
Amy Gravely indicated no relevant financial relationships.
Khalid Ishani indicated no relevant financial relationships.
Rahul Karna indicated no relevant financial relationships.
Aasma Shaukat: Freenome inc – Consultant.
Natalie J. Wilson, MD1, Mohammad Bilal, MD, FACG2, Anders Westanmo, PharmD, MBA3, Amy Gravely, MA4, Khalid Ishani, BS5, Rahul Karna, MD6, Aasma Shaukat, MD, MPH, FACG7, 9, Yield of Post-Polypectomy Interval Fecal Immunochemical Testing: Results From a Large Nationwide Veterans’ Affairs Database, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1University of Minnesota, Minneapolis, MN; 2University of Colorado Anschutz Medical Campus, Denver, CO; 3Minneapolis VA Health Care System, Minneapolis, MN; 4Minneapolis VA Medical Center, Research Service, Minneapolis, MN; 5University of Minnesota Medical School, Minneapolis, MN; 6University of Minnesota Medical Center, Minneapolis, MN; 7NYU Grossman School of Medicine, Division of Gastroenterology and Hepatology, New York, NY
Introduction: Current guidelines recommend surveillance colonoscopy after polypectomy. In real-world practice, fecal immunochemical testing (FIT) is sometimes used for surveillance after polypectomy, though little data exists to inform its use in this setting.
This study aims to evaluate the yield of interval FIT (iFIT) in patients with prior polypectomy compared to those without polypectomy, focusing on 1) the impact of polypectomy on iFIT results, 2) adherence to follow-up colonoscopy after a positive iFIT, and 3) to determine whether polypectomy is a predictor of advanced colorectal neoplasia (ACRN) or CRC after positive iFIT.
Methods: We analyzed data from the Veterans Health Administration Corporate Data Warehouse from 2000-2024. Patients who underwent colonoscopy for any indication followed by iFIT within 10 years and before another colonoscopy were included. Advanced colorectal neoplasia (ACRN) was defined as adenoma with high-grade dysplasia (HGD), tubulovillous (TVA), or CRC.
Results: Overall, 4,799,644 individuals underwent colonoscopy and 10.9% (525,157) had iFIT. Of these, 49.9% underwent polypectomy at index colonoscopy. Demographics and index colonoscopy findings are shown in Table 1.
The average time from index colonoscopy to iFIT was 5.9 years. iFIT was positive in 17.2% (44,931) of post-polypectomy patients vs 14.1% (37,081) without polypectomy. History of polypectomy was an independent predictor of positive iFIT (OR 1.12; 95% CI, 1.10–1.15; p< 0.0001). Follow-up colonoscopy after positive iFIT was performed in 50.4% of post-polypectomy patients and 49.3% without polypectomy. Findings on follow-up colonoscopy in those with and without polypectomy were as follows: tubular adenoma (54.7% vs 45.8%), TVA (5.6% vs 4.7%), adenoma with HGD (0.82% vs 0.66%), sessile serrated lesion (3.52% vs 2.42%), ACRN (9.2% vs 7.9%), and CRC (3.3% vs 3.0%), respectively (Table 2). Prior polypectomy was not an independent predictor of CRC (OR 0.96; 95% CI, 0.82–1.13; p=0.65) or ACRN (OR 0.97; 95% CI, 0.88–1.07; p=0.57) at colonoscopy post-iFIT.
Discussion: In this large US cohort, iFIT was performed frequently in patients with and without polypectomy at index colonoscopy, and was positive in 14-17%. Colonoscopy after positive iFIT revealed high rates of ACRN and CRC, irrespective of polypectomy history. These findings reinforce the importance of colonoscopy following positive iFIT given the high risk of ACRN and CRC, regardless of polypectomy history.

Figure: Table 1. Demographics and index colonoscopy findings, stratified by polypectomy history at index colonoscopy

Figure: Table 2. Findings on colonoscopy performed after a positive interval FIT, stratified by history of polypectomy at index colonoscopy
Disclosures:
Natalie Wilson indicated no relevant financial relationships.
Mohammad Bilal: Boston Scientific – Consultant. Cook endoscopy – Paid speaker. Steris Endoscopy – Consultant.
Anders Westanmo indicated no relevant financial relationships.
Amy Gravely indicated no relevant financial relationships.
Khalid Ishani indicated no relevant financial relationships.
Rahul Karna indicated no relevant financial relationships.
Aasma Shaukat: Freenome inc – Consultant.
Natalie J. Wilson, MD1, Mohammad Bilal, MD, FACG2, Anders Westanmo, PharmD, MBA3, Amy Gravely, MA4, Khalid Ishani, BS5, Rahul Karna, MD6, Aasma Shaukat, MD, MPH, FACG7, 9, Yield of Post-Polypectomy Interval Fecal Immunochemical Testing: Results From a Large Nationwide Veterans’ Affairs Database, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.



