Oral Paper Presentation
Annual Scientific Meeting
Session: Presidential Plenary Session 2
7 - Treatment With Resmetirom for up to Two Years Led to Improvement in Liver Stiffness, Fibrosis Biomarkers, Fibrosis Scores, and Portal Hypertension Risk in 122 Patients With Compensated MASH Cirrhosis
Monday, October 27, 2025
9:42 AM - 9:54 AM PDT
Location: North Ballroom 120D
.jpg)
Naim Alkhouri, MD (he/him/his)
Chief Academic Officer. Director, Steatotic Liver Disease Program
Summit Clinical Research
Phoenix, AZ
Presenting Author(s)
Award: ACG Governors Award for Excellence in Clinical Research
Naim Alkhouri, MD, MHSc1, Rebecca Taub, MD2, Dominic Labriola, PhD2, Xiaomin Lu, PhD2, Sam Moussa, MD3, Mazen Noureddin, MD4
1Arizona Liver Health, Phoenix, AZ; 2Madrigal Pharmaceuticals, West Conshohocken, PA; 3University of Arizona College of Medicine, Phoenix, AZ; 4Houston Research Institute and Houston Methodist Hospital, Houston, TX
Introduction: Resmetirom, a selective thyroid hormone receptor beta agonist, is an approved therapy for metabolic dysfunction-associated steatohepatitis (MASH) with moderate to advanced liver fibrosis based on improvement in both NASH and fibrosis. MASH cirrhosis with clinically significant portal hypertension (CSPH) leads to major adverse liver outcomes. This analysis aimed to assess the effect of resmetirom over two years of treatment in 122 patients with MASH cirrhosis, with and without CSPH, as defined by Baveno VII criteria.
Methods: A total of 122 patients with Child Pugh A MASH cirrhosis (based on MASH F4 on historic biopsy >66% or clinical diagnosis) were treated with 80 mg resmetirom for up to 2 years (MAESTRO- NAFLD-1 (NCT04197479) year 1; open-label extension trial (NCT04951219) (year 2)). Patients were assessed for baseline CSPH (Baveno VII) with FibroScan vibration-controlled transient elastography (VCTE), platelet count and confirmed using magnetic resonance elastography (MRE). Non-invasive biomarkers and imaging were analysed at baseline and out to 2 years. Results are presented as mean change or % change from baseline.
Results: Baseline characteristics and outcomes (changes from baseline) are listed in the Table. At baseline, 63% of patients were categorized as probable/definitive CSPH (Baveno VII), and at 1 and 2 years, respectively, 20% and 28% of CSPH positive patients no longer met criteria for CSPH. 35% of patients with confirmed F4 at baseline (liver biopsy F4 and/or platelets < 140/MRE≥5 with VCTE≥15) showed a transition from F4 to F3 at year 2 (VCTE< 15 and ≥25% decrease from baseline). Discontinuation rate was 8%. Mild gastrointestinal disorders were the most common adverse events.
Discussion: At 2 years of treatment, resmetirom demonstrated significant improvements in non- invasive biomarkers, liver stiffness on imaging and portal hypertension risk in patients with MASH cirrhosis. Resmetirom was safe and well-tolerated in this population. These findings highlight the potential of resmetirom to demonstrate clinical benefit in MAESTRO-NASH OUTCOMES, an ongoing 845 MASH cirrhosis patient clinical outcome study.
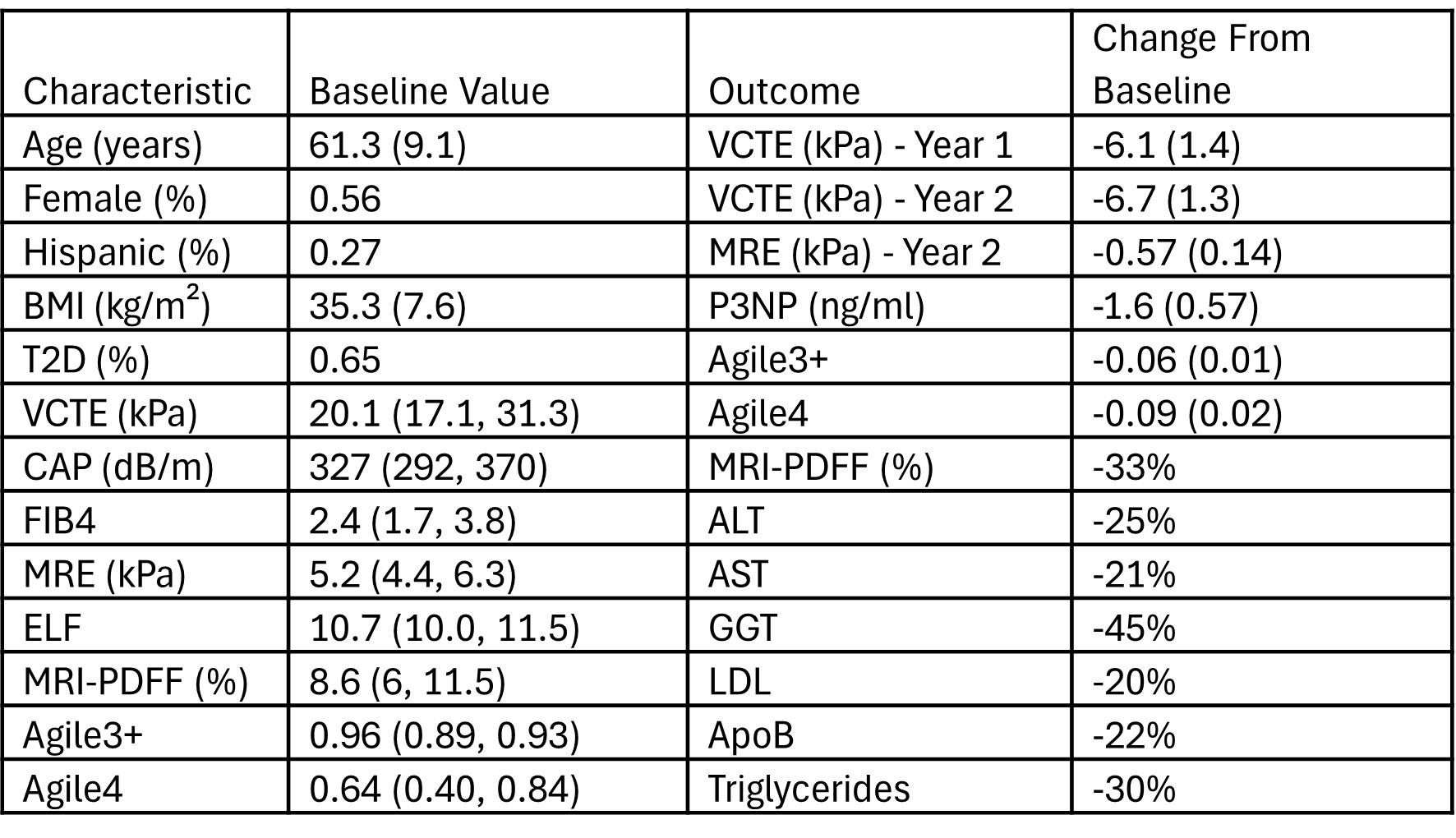
Figure: Baseline characteristics and outcome changes from baseline
Disclosures:
Naim Alkhouri: Madrigal Pharmaceuticals – Advisor or Review Panel Member, Consultant, Speakers Bureau.
Rebecca Taub: Madrigal Pharmaceuticals – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Dominic Labriola: Madrigal Pharmaceuticals – Employee, Stock Options.
Xiaomin Lu: Madrigal Pharmaceuticals – Employee, Stock Options.
Sam Moussa: Madrigal Pharmaceuticals – Consultant.
Mazen Noureddin: Madrigal Pharmaceuticals – Advisor or Review Panel Member, Consultant, Royalties, Speakers Bureau.
Naim Alkhouri, MD, MHSc1, Rebecca Taub, MD2, Dominic Labriola, PhD2, Xiaomin Lu, PhD2, Sam Moussa, MD3, Mazen Noureddin, MD4, 7, Treatment With Resmetirom for up to Two Years Led to Improvement in Liver Stiffness, Fibrosis Biomarkers, Fibrosis Scores, and Portal Hypertension Risk in 122 Patients With Compensated MASH Cirrhosis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
Naim Alkhouri, MD, MHSc1, Rebecca Taub, MD2, Dominic Labriola, PhD2, Xiaomin Lu, PhD2, Sam Moussa, MD3, Mazen Noureddin, MD4
1Arizona Liver Health, Phoenix, AZ; 2Madrigal Pharmaceuticals, West Conshohocken, PA; 3University of Arizona College of Medicine, Phoenix, AZ; 4Houston Research Institute and Houston Methodist Hospital, Houston, TX
Introduction: Resmetirom, a selective thyroid hormone receptor beta agonist, is an approved therapy for metabolic dysfunction-associated steatohepatitis (MASH) with moderate to advanced liver fibrosis based on improvement in both NASH and fibrosis. MASH cirrhosis with clinically significant portal hypertension (CSPH) leads to major adverse liver outcomes. This analysis aimed to assess the effect of resmetirom over two years of treatment in 122 patients with MASH cirrhosis, with and without CSPH, as defined by Baveno VII criteria.
Methods: A total of 122 patients with Child Pugh A MASH cirrhosis (based on MASH F4 on historic biopsy >66% or clinical diagnosis) were treated with 80 mg resmetirom for up to 2 years (MAESTRO- NAFLD-1 (NCT04197479) year 1; open-label extension trial (NCT04951219) (year 2)). Patients were assessed for baseline CSPH (Baveno VII) with FibroScan vibration-controlled transient elastography (VCTE), platelet count and confirmed using magnetic resonance elastography (MRE). Non-invasive biomarkers and imaging were analysed at baseline and out to 2 years. Results are presented as mean change or % change from baseline.
Results: Baseline characteristics and outcomes (changes from baseline) are listed in the Table. At baseline, 63% of patients were categorized as probable/definitive CSPH (Baveno VII), and at 1 and 2 years, respectively, 20% and 28% of CSPH positive patients no longer met criteria for CSPH. 35% of patients with confirmed F4 at baseline (liver biopsy F4 and/or platelets < 140/MRE≥5 with VCTE≥15) showed a transition from F4 to F3 at year 2 (VCTE< 15 and ≥25% decrease from baseline). Discontinuation rate was 8%. Mild gastrointestinal disorders were the most common adverse events.
Discussion: At 2 years of treatment, resmetirom demonstrated significant improvements in non- invasive biomarkers, liver stiffness on imaging and portal hypertension risk in patients with MASH cirrhosis. Resmetirom was safe and well-tolerated in this population. These findings highlight the potential of resmetirom to demonstrate clinical benefit in MAESTRO-NASH OUTCOMES, an ongoing 845 MASH cirrhosis patient clinical outcome study.

Figure: Baseline characteristics and outcome changes from baseline
Disclosures:
Naim Alkhouri: Madrigal Pharmaceuticals – Advisor or Review Panel Member, Consultant, Speakers Bureau.
Rebecca Taub: Madrigal Pharmaceuticals – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Dominic Labriola: Madrigal Pharmaceuticals – Employee, Stock Options.
Xiaomin Lu: Madrigal Pharmaceuticals – Employee, Stock Options.
Sam Moussa: Madrigal Pharmaceuticals – Consultant.
Mazen Noureddin: Madrigal Pharmaceuticals – Advisor or Review Panel Member, Consultant, Royalties, Speakers Bureau.
Naim Alkhouri, MD, MHSc1, Rebecca Taub, MD2, Dominic Labriola, PhD2, Xiaomin Lu, PhD2, Sam Moussa, MD3, Mazen Noureddin, MD4, 7, Treatment With Resmetirom for up to Two Years Led to Improvement in Liver Stiffness, Fibrosis Biomarkers, Fibrosis Scores, and Portal Hypertension Risk in 122 Patients With Compensated MASH Cirrhosis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.




