Oral Paper Presentation
Annual Scientific Meeting
Session: Presidential Plenary Session 1
4 - Efficacy and Safety of Subcutaneous Guselkumab Induction and Maintenance Therapy in Participants With Ulcerative Colitis: Results Through Week 48 From the Phase 3 ASTRO Study
Monday, October 27, 2025
8:36 AM - 8:48 AM PDT
Location: North Ballroom 120D

Jessica R. Allegretti, MD, MPH, FACG (she/her/hers)
Division of Gastroenterology, Hepatology, and Endoscopy, Brigham and Women’s Hospital, Harvard Medical School
Boston, MA
Presenting Author(s)
Jessica R.. Allegretti, MD, MPH, FACG1, Laurent Peyrin-Biroulet, MD, PhD2, Millie D. Long, MD, FACG3, Matthew Germinaro, MD4, Thomas Baker, MD4, Mary Kavalam, 4, Yelina Alvarez, 4, Karen Hertzog, 4, Silke Jorgens, 5, Hongyan Zhang, 4, Lingjing Jiang, 4, Tadakazu Hisamatsu, MD, PhD6, David T. Rubin, MD7, Silvio Danese, MD, PhD8
1Division of Gastroenterology, Hepatology, and Endoscopy, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA; 2Department of Gastroenterology, CHRU Nancy, INSERM NGERE, Université de Lorraine, France, Vandœuvre-lès-Nancy, Lorraine, France; 3Division of Gastroenterology and Hepatology, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA, Chapel Hill, NC; 4Johnson & Johnson, Spring House, PA; 5Johnson & Johnson, Neuss, Nordrhein-Westfalen, Germany; 6Department of Gastroenterology and Hepatology, Kyorin University School of Medicine, Tokyo, Tokyo, Japan; 7University of Chicago Medicine Inflammatory Bowel Disease Center, Chicago, IL; 8Gastroenterology and Endoscopy, IRCCS Ospedale San Raffaele and University Vita-Salute San Raffaele, Milan, Lombardia, Italy
Introduction: Guselkumab (GUS), a dual-acting interleukin-23p19 subunit inhibitor, demonstrated efficacy in participants (pts) with ulcerative colitis (UC) treated with intravenous (IV) or subcutaneous (SC) induction. Here, we report the efficacy and safety of SC induction followed by SC maintenance through Week (W) 48 in ASTRO, a phase 3, randomized, double-blind, placebo (PBO)-controlled, treat-through study in pts with moderately to severely active UC.
Methods: Eligible pts had modified Mayo scores of 5 to 9, rectal bleeding subscore of ≥1, and Mayo endoscopic subscore (MES) ≥2. Pts also had documented inadequate response/intolerance to biologics, Janus kinase (JAK) inhibitors, and/or sphingosine-1-phosphate (S1P) inhibitors (BIO/JAKi/S1Pi-IR) or to corticosteroids, 6-mercaptopurine, or azathioprine. Randomization was stratified by baseline BIO/JAKi/S1Pi-IR status and MES with 418 pts allocated 1:1:1 to GUS 400 mg SC every 4 weeks (q4w) (×3)→GUS 200 mg SC q4w (N=140), GUS 400 mg SC q4w (×3)→GUS 100 mg SC every 8 weeks (q8w) (N=139), or PBO SC (N=139). PBO pts who met rescue criteria were switched to GUS at W16; GUS pts who met rescue criteria stayed on their assigned GUS dose regimen (sham rescue).
Results: At W48, greater proportions of pts in the GUS groups achieved clinical remission compared with PBO (36.7% for GUS 100 mg q8w [Δ 29.5%], 42.9% for GUS 200 mg q4w [Δ 35.6%], 7.2% for PBO) (Figure). Endoscopic remission proportions were 25.9% for GUS 100 mg q8w (Δ 20.9%), 26.4% for GUS 200 mg q4w (Δ 21.4%), and 5.0% for PBO. Clinical response, symptomatic remission, endoscopic improvement, histologic improvement, and histologic remission proportions were also greater for GUS vs PBO. Consistent with the results of the overall population, efficacy was also observed for subpopulations of BIO/JAKi/S1Pi-naïve and BIO/JAKi/S1Pi-IR pts. Safety events per 100 pts-years were not greater in the GUS groups compared with the PBO group: adverse events (AE) (GUS 100 mg q8w and 200 mg q4w vs PBO: 308.3 and 310.7 vs 357.1), serious AEs (6.5 and 8.2 vs 38.4), serious infections (0.8 and 2.5 vs 3.7), and AEs leading to treatment discontinuation (4.9 and 4.1 vs 19.8).
Discussion: GUS SC induction followed by SC maintenance was efficacious through 1 year in pts with moderately to severely active UC, and the safety profile is consistent with GUS in its approved indications including UC.
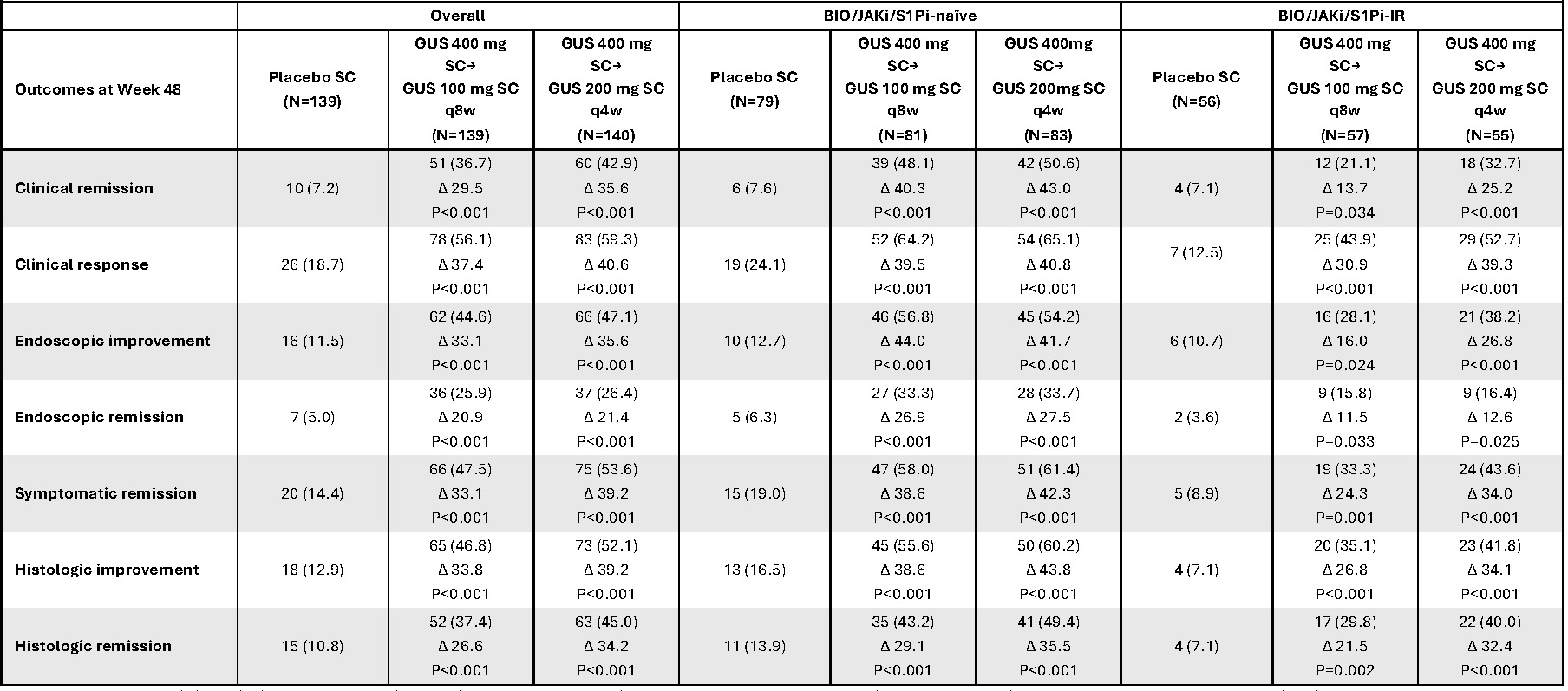
Figure:
Title: Efficacy endpoints at Week 48 in the overall population and by biologic, JAK inhibitor, and/or S1P inhibitor history
Caption: Data are presented as n (%); ∆% (adjusted treatment difference) versus placebo; nominal p-value versus placebo. The adjusted treatment difference was based on the common risk difference by use of the Mantel-Haenszel stratum weights and the Sato variance estimator. In the overall population, the p-values were based on the Mantel-Haenszel test, stratified by BIO/JAKi/S1Pi-IR status (Yes/No) and Mayo endoscopic subscore at baseline (Moderate [2]/Severe [3]). For the BIO/JAKi/S1Pi-naive/-IR subgroups, the p-values were stratified only by the Mayo endoscopic subscore at baseline. Participants who, prior to Week 48, had an ostomy or colectomy, a prohibited change in UC medications, discontinued study agent due to lack of efficacy or an AE of worsening of UC, or met rescue criteria per IWRS were considered not to have achieved any of the key efficacy endpoints shown at Week 48. For participants who discontinued study agent due to COVID-19-related reasons (excluding COVID-19 infection) or regional crisis, their observed values were used, if available. Participants who discontinued study agent prior to Week 48 due to other reasons were considered not to have achieved any of the efficacy endpoints shown at Week 48. Participants who were missing one or more of the components pertaining to an endpoint at Week 48 were considered not to have achieved the endpoint.
Endpoint definitions: clinical remission, Mayo SFS 0/1 and not increased from BL, Mayo RBS=0, and MES 0/1 with no friability; clinical response, ≥30% and ≥2-point decrease from BL in Modified Mayo score with ≥1-point decrease from BL in RBS or RBS 0/1; endoscopic improvement, MES 0/1 with no friability; endoscopic remission, MES of 0; symptomatic remission, SFS 0/1 and not increased from BL and RBS=0; histologic improvement, neutrophil infiltration in <5% of crypts; no crypt destruction; and no erosions, ulcerations, or granulation tissue per Geboes grading system; histologic remission, absence of neutrophils from the mucosa (both lamina propria and epithelium); no crypt destruction; and no erosions, ulcerations, or granulation tissue per Geboes grading system.
AE=adverse event; BIO=biologic; BL=baseline; COVID-19=coronavirus disease 2019; GUS=guselkumab; IR=inadequate response; IWRS=Interactive Web Response System; JAK=Janus kinase; JAKi=JAK inhibitor; MES=Mayo endoscopic subscore; N=population size; n=sample size; q4w=every 4 weeks; q8w=every 8 weeks; RBS=rectal bleeding subscore; S1P= sphingosine-1-phosphate; S1Pi=S1P inhibitor; SC=subcutaneous; SFS=stool frequency subscore; UC=ulcerative colitis
Disclosures:
Jessica Allegretti: Abbvie – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Speaker, Speakers Bureau. Adiso – Consultant. Bristol Myer Squibb – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Speaker. Celltrion – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. Ferring – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. Finch – Consultant. Genentech – Advisory Committee/Board Member, Consultant. GlaxoSmithKline – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. Iterative Scopes – Consultant. Janssen – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Speakers Bureau. Johnson & Johnson – Consultant, Grant/Research Support, Speaker. Merck – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Grant/Research Support. Pfizer – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Grant/Research Support. Roivant – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. Roivant Adiso – Consultant. Seres Therapeutics – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. Shattuck Labs – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. TRXBio – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. Vedanta – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant.
Laurent Peyrin-Biroulet: AbbVie – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, meeting attendance/travel support, Speakers Bureau. Abivax – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Adacyte – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. Alfasigma – Speakers Bureau. Alimentiv – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Amgen – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, meeting attendance/travel support, Speakers Bureau. Applied Molecular Transport – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Arena – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, Speakers Bureau. Banook – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Biogen – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, Speakers Bureau. Bristol Myers Squibb – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Celltrion – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Grant/Research Support, Honoraria, meeting attendance/travel support, Speakers Bureau. Connect Biopharm – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, meeting attendance/travel support. Cytoki Pharma – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Eli Lilly – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, meeting attendance/travel support, Speakers Bureau. Enthera – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. F. Hoffmann-La Roche Ltd – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Ferring – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, meeting attendance/travel support, Speakers Bureau. Fresenius Kabi – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Grant/Research Support, Honoraria. Galapagos – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, meeting attendance/travel support, Speakers Bureau. Genentech – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, meeting attendance/travel support, Speakers Bureau. Gilead – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, meeting attendance/travel support, Speakers Bureau. Gossamer Bio – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, meeting attendance/travel support. GSK – Advisory Committee/Board Member, Consultant. IAC Image Analysis – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Index Pharmaceuticals – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Inotrem – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Janssen – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Speakers Bureau. Johnson & Johnson – Advisory Committee/Board Member, Consultant, Honoraria, meeting attendance/travel support. Medac – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Grant/Research Support, Honoraria, meeting attendance/travel support, Speakers Bureau. Mopac – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Morphic – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, meeting attendance/travel support. MSD – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Grant/Research Support, Honoraria, meeting attendance/travel support, Speakers Bureau. Nordic Pharma – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, Speakers Bureau. Novartis – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Oncodesign Precision Medicine – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. ONO Pharma – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. OSE Immunotherapeutics – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Pandion Therapeutics – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Par' Immune – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Pfizer – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, meeting attendance/travel support, Speakers Bureau. Prometheus – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Protagonist – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Samsung – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Sandoz – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, meeting attendance/travel support, Speakers Bureau. Sanofi – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Satisfay – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Takeda – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Grant/Research Support, Honoraria, meeting attendance/travel support, Speakers Bureau. Telavant – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Theravance – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Thermo Fischer – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, meeting attendance/travel support. Tigenix – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Tillots – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, meeting attendance/travel support, Speakers Bureau. Vectivbio – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Ventyx – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Viatris – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, Speakers Bureau. Ysopia – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria.
Millie Long: AbbVie – Consultant. Bristol Myers Squibb – Consultant. Celltrion – Consultant, Grant/Research Support. Intercept – Consultant. Johnson & Johnson – Consultant. Lilly – Consultant, Grant/Research Support. Merck – Consultant. Pfizer – Consultant, Grant/Research Support. Prometheus – Consultant. Roivant – Consultant. Sanofi – Consultant. Spyre – Consultant. Takeda – Consultant, Grant/Research Support. Target RWE – Consultant.
Matthew Germinaro: Johnson & Johnson – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Thomas Baker: Johnson & Johnson – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Mary Kavalam: Johnson & Johnson – Employee, Stock Options.
Yelina Alvarez: Johnson & Johnson – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Karen Hertzog: Johnson & Johnson – Employee. Johnson & Johnson – Employee. Johnson & Johnson – Stock Options, Stock-publicly held company(excluding mutual/index funds).
Silke Jorgens: Johnson & Johnson – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Hongyan Zhang: Johnson & Johnson – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Lingjing Jiang: Johnson & Johnson – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Tadakazu Hisamatsu: AbbVie GK – Consultant, Grant/Research Support, Lecture fees. Boston Scientific Corporation – Grant/Research Support. Bristol Myers Squibb – Consultant. Daiichi Sankyo Co. Ltd. – Grant/Research Support, Honararium. EA Pharma Co. Ltd. – Consultant, Grant/Research Support, Lecture fees. Gilead Sciences – Consultant. JIMRO Co. Ltd. – Grant/Research Support, Lecture fees. Johnson & Johnson – Consultant, Lecture fees. Kissei Pharmaceutical Co. Ltd. – Grant/Research Support, Lecture fees. Kyorin Pharmaceutical Co. Ltd. – Grant/Research Support, Lecture fees. Lilly – Consultant. Mitsubishi Tanabe Pharma Corporation – Consultant, Grant/Research Support, Lecture fees. Mochida Pharmaceutical Co. Ltd. – Grant/Research Support, Lecture fees. Nichi-Iko Pharmaceutical Co. Ltd – Consultant. Nippon Kayaku Co. Ltd. – Grant/Research Support. Pfizer Inc. – Consultant, Grant/Research Support, Lecture fees. Takeda Pharmaceutical Co. Ltd. – Grant/Research Support, Lecture fees. Zeria Pharmaceutical Co – Grant/Research Support.
David Rubin: AbbVie – Advisory Committee/Board Member, Consultant, Speakers Bureau. Altrubio – Advisory Committee/Board Member, Consultant, Speakers Bureau, Stock Options. Apex – Advisory Committee/Board Member, Consultant, Speakers Bureau. Avalo – Advisory Committee/Board Member, Consultant, Speakers Bureau. Bristol Myers Squibb – Advisory Committee/Board Member, Consultant, Speakers Bureau. Buhlmann Diagnostics – Advisory Committee/Board Member, Consultant, Speakers Bureau. Celgene – Advisory Committee/Board Member, Consultant, Speakers Bureau. Connect BioPharma – Advisory Committee/Board Member, Consultant, Speakers Bureau. Cornerstones Health, Inc. – Advisory Committee/Board Member. Crohn's & Colitis Foundation – Advisory Committee/Board Member. Datos Health – Stock Options. Intouch Group – Advisory Committee/Board Member, Consultant, Speakers Bureau. Iterative Health – Advisory Committee/Board Member, Consultant, Speakers Bureau, Stock Options. Johnson & Johnson – Advisory Committee/Board Member, Consultant, Speakers Bureau. Lilly – Advisory Committee/Board Member, Consultant, Speakers Bureau. Pfizer – Advisory Committee/Board Member, Consultant, Speakers Bureau. Samsung Neurologica – Advisory Committee/Board Member, Consultant, Speakers Bureau. Takeda – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau.
Silvio Danese: AbbVie – Consultant, Lecture fees. Alimentiv – Consultant. Allergan – Consultant. Amgen – Consultant, Lecture fees. AstraZeneca – Consultant. Athos Therapeutics – Consultant. Biogen – Consultant. Boehringer Ingelheim – Consultant. Celgene – Consultant. Celltrion – Consultant. Eli Lilly – Consultant. Enthera – Consultant. F. Hoffmann-La Roche Ltd – Consultant. Ferring Pharmaceuticals Inc. – Consultant, Lecture fees. Gilead – Consultant, Lecture fees. Hospira – Consultant. Inotrem – Consultant. Johnson & Johnson – Consultant, Lecture fees. MSD – Consultant. Mundipharma – Consultant. Mylan – Consultant, Lecture fees. Pfizer – Consultant, Lecture fees. Sandoz – Consultant. Sublimity Therapeutics – Consultant. Takeda – Consultant, Lecture fees. TiGenix – Consultant. UCB Inc. – Consultant. Vifor (International) Ltd. – Consultant.
Jessica R.. Allegretti, MD, MPH, FACG1, Laurent Peyrin-Biroulet, MD, PhD2, Millie D. Long, MD, FACG3, Matthew Germinaro, MD4, Thomas Baker, MD4, Mary Kavalam, 4, Yelina Alvarez, 4, Karen Hertzog, 4, Silke Jorgens, 5, Hongyan Zhang, 4, Lingjing Jiang, 4, Tadakazu Hisamatsu, MD, PhD6, David T. Rubin, MD7, Silvio Danese, MD, PhD8, 4, Efficacy and Safety of Subcutaneous Guselkumab Induction and Maintenance Therapy in Participants With Ulcerative Colitis: Results Through Week 48 From the Phase 3 ASTRO Study, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Division of Gastroenterology, Hepatology, and Endoscopy, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA; 2Department of Gastroenterology, CHRU Nancy, INSERM NGERE, Université de Lorraine, France, Vandœuvre-lès-Nancy, Lorraine, France; 3Division of Gastroenterology and Hepatology, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA, Chapel Hill, NC; 4Johnson & Johnson, Spring House, PA; 5Johnson & Johnson, Neuss, Nordrhein-Westfalen, Germany; 6Department of Gastroenterology and Hepatology, Kyorin University School of Medicine, Tokyo, Tokyo, Japan; 7University of Chicago Medicine Inflammatory Bowel Disease Center, Chicago, IL; 8Gastroenterology and Endoscopy, IRCCS Ospedale San Raffaele and University Vita-Salute San Raffaele, Milan, Lombardia, Italy
Introduction: Guselkumab (GUS), a dual-acting interleukin-23p19 subunit inhibitor, demonstrated efficacy in participants (pts) with ulcerative colitis (UC) treated with intravenous (IV) or subcutaneous (SC) induction. Here, we report the efficacy and safety of SC induction followed by SC maintenance through Week (W) 48 in ASTRO, a phase 3, randomized, double-blind, placebo (PBO)-controlled, treat-through study in pts with moderately to severely active UC.
Methods: Eligible pts had modified Mayo scores of 5 to 9, rectal bleeding subscore of ≥1, and Mayo endoscopic subscore (MES) ≥2. Pts also had documented inadequate response/intolerance to biologics, Janus kinase (JAK) inhibitors, and/or sphingosine-1-phosphate (S1P) inhibitors (BIO/JAKi/S1Pi-IR) or to corticosteroids, 6-mercaptopurine, or azathioprine. Randomization was stratified by baseline BIO/JAKi/S1Pi-IR status and MES with 418 pts allocated 1:1:1 to GUS 400 mg SC every 4 weeks (q4w) (×3)→GUS 200 mg SC q4w (N=140), GUS 400 mg SC q4w (×3)→GUS 100 mg SC every 8 weeks (q8w) (N=139), or PBO SC (N=139). PBO pts who met rescue criteria were switched to GUS at W16; GUS pts who met rescue criteria stayed on their assigned GUS dose regimen (sham rescue).
Results: At W48, greater proportions of pts in the GUS groups achieved clinical remission compared with PBO (36.7% for GUS 100 mg q8w [Δ 29.5%], 42.9% for GUS 200 mg q4w [Δ 35.6%], 7.2% for PBO) (Figure). Endoscopic remission proportions were 25.9% for GUS 100 mg q8w (Δ 20.9%), 26.4% for GUS 200 mg q4w (Δ 21.4%), and 5.0% for PBO. Clinical response, symptomatic remission, endoscopic improvement, histologic improvement, and histologic remission proportions were also greater for GUS vs PBO. Consistent with the results of the overall population, efficacy was also observed for subpopulations of BIO/JAKi/S1Pi-naïve and BIO/JAKi/S1Pi-IR pts. Safety events per 100 pts-years were not greater in the GUS groups compared with the PBO group: adverse events (AE) (GUS 100 mg q8w and 200 mg q4w vs PBO: 308.3 and 310.7 vs 357.1), serious AEs (6.5 and 8.2 vs 38.4), serious infections (0.8 and 2.5 vs 3.7), and AEs leading to treatment discontinuation (4.9 and 4.1 vs 19.8).
Discussion: GUS SC induction followed by SC maintenance was efficacious through 1 year in pts with moderately to severely active UC, and the safety profile is consistent with GUS in its approved indications including UC.

Figure:
Title: Efficacy endpoints at Week 48 in the overall population and by biologic, JAK inhibitor, and/or S1P inhibitor history
Caption: Data are presented as n (%); ∆% (adjusted treatment difference) versus placebo; nominal p-value versus placebo. The adjusted treatment difference was based on the common risk difference by use of the Mantel-Haenszel stratum weights and the Sato variance estimator. In the overall population, the p-values were based on the Mantel-Haenszel test, stratified by BIO/JAKi/S1Pi-IR status (Yes/No) and Mayo endoscopic subscore at baseline (Moderate [2]/Severe [3]). For the BIO/JAKi/S1Pi-naive/-IR subgroups, the p-values were stratified only by the Mayo endoscopic subscore at baseline. Participants who, prior to Week 48, had an ostomy or colectomy, a prohibited change in UC medications, discontinued study agent due to lack of efficacy or an AE of worsening of UC, or met rescue criteria per IWRS were considered not to have achieved any of the key efficacy endpoints shown at Week 48. For participants who discontinued study agent due to COVID-19-related reasons (excluding COVID-19 infection) or regional crisis, their observed values were used, if available. Participants who discontinued study agent prior to Week 48 due to other reasons were considered not to have achieved any of the efficacy endpoints shown at Week 48. Participants who were missing one or more of the components pertaining to an endpoint at Week 48 were considered not to have achieved the endpoint.
Endpoint definitions: clinical remission, Mayo SFS 0/1 and not increased from BL, Mayo RBS=0, and MES 0/1 with no friability; clinical response, ≥30% and ≥2-point decrease from BL in Modified Mayo score with ≥1-point decrease from BL in RBS or RBS 0/1; endoscopic improvement, MES 0/1 with no friability; endoscopic remission, MES of 0; symptomatic remission, SFS 0/1 and not increased from BL and RBS=0; histologic improvement, neutrophil infiltration in <5% of crypts; no crypt destruction; and no erosions, ulcerations, or granulation tissue per Geboes grading system; histologic remission, absence of neutrophils from the mucosa (both lamina propria and epithelium); no crypt destruction; and no erosions, ulcerations, or granulation tissue per Geboes grading system.
AE=adverse event; BIO=biologic; BL=baseline; COVID-19=coronavirus disease 2019; GUS=guselkumab; IR=inadequate response; IWRS=Interactive Web Response System; JAK=Janus kinase; JAKi=JAK inhibitor; MES=Mayo endoscopic subscore; N=population size; n=sample size; q4w=every 4 weeks; q8w=every 8 weeks; RBS=rectal bleeding subscore; S1P= sphingosine-1-phosphate; S1Pi=S1P inhibitor; SC=subcutaneous; SFS=stool frequency subscore; UC=ulcerative colitis
Disclosures:
Jessica Allegretti: Abbvie – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Speaker, Speakers Bureau. Adiso – Consultant. Bristol Myer Squibb – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Speaker. Celltrion – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. Ferring – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. Finch – Consultant. Genentech – Advisory Committee/Board Member, Consultant. GlaxoSmithKline – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. Iterative Scopes – Consultant. Janssen – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Speakers Bureau. Johnson & Johnson – Consultant, Grant/Research Support, Speaker. Merck – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Grant/Research Support. Pfizer – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Grant/Research Support. Roivant – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. Roivant Adiso – Consultant. Seres Therapeutics – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. Shattuck Labs – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. TRXBio – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. Vedanta – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant.
Laurent Peyrin-Biroulet: AbbVie – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, meeting attendance/travel support, Speakers Bureau. Abivax – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Adacyte – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. Alfasigma – Speakers Bureau. Alimentiv – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Amgen – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, meeting attendance/travel support, Speakers Bureau. Applied Molecular Transport – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Arena – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, Speakers Bureau. Banook – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Biogen – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, Speakers Bureau. Bristol Myers Squibb – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Celltrion – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Grant/Research Support, Honoraria, meeting attendance/travel support, Speakers Bureau. Connect Biopharm – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, meeting attendance/travel support. Cytoki Pharma – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Eli Lilly – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, meeting attendance/travel support, Speakers Bureau. Enthera – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. F. Hoffmann-La Roche Ltd – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Ferring – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, meeting attendance/travel support, Speakers Bureau. Fresenius Kabi – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Grant/Research Support, Honoraria. Galapagos – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, meeting attendance/travel support, Speakers Bureau. Genentech – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, meeting attendance/travel support, Speakers Bureau. Gilead – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, meeting attendance/travel support, Speakers Bureau. Gossamer Bio – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, meeting attendance/travel support. GSK – Advisory Committee/Board Member, Consultant. IAC Image Analysis – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Index Pharmaceuticals – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Inotrem – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Janssen – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Speakers Bureau. Johnson & Johnson – Advisory Committee/Board Member, Consultant, Honoraria, meeting attendance/travel support. Medac – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Grant/Research Support, Honoraria, meeting attendance/travel support, Speakers Bureau. Mopac – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Morphic – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, meeting attendance/travel support. MSD – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Grant/Research Support, Honoraria, meeting attendance/travel support, Speakers Bureau. Nordic Pharma – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, Speakers Bureau. Novartis – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Oncodesign Precision Medicine – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. ONO Pharma – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. OSE Immunotherapeutics – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Pandion Therapeutics – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Par' Immune – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Pfizer – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, meeting attendance/travel support, Speakers Bureau. Prometheus – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Protagonist – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Samsung – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Sandoz – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, meeting attendance/travel support, Speakers Bureau. Sanofi – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Satisfay – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Takeda – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Grant/Research Support, Honoraria, meeting attendance/travel support, Speakers Bureau. Telavant – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Theravance – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Thermo Fischer – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, meeting attendance/travel support. Tigenix – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Tillots – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, meeting attendance/travel support, Speakers Bureau. Vectivbio – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Ventyx – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria. Viatris – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria, Speakers Bureau. Ysopia – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Honoraria.
Millie Long: AbbVie – Consultant. Bristol Myers Squibb – Consultant. Celltrion – Consultant, Grant/Research Support. Intercept – Consultant. Johnson & Johnson – Consultant. Lilly – Consultant, Grant/Research Support. Merck – Consultant. Pfizer – Consultant, Grant/Research Support. Prometheus – Consultant. Roivant – Consultant. Sanofi – Consultant. Spyre – Consultant. Takeda – Consultant, Grant/Research Support. Target RWE – Consultant.
Matthew Germinaro: Johnson & Johnson – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Thomas Baker: Johnson & Johnson – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Mary Kavalam: Johnson & Johnson – Employee, Stock Options.
Yelina Alvarez: Johnson & Johnson – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Karen Hertzog: Johnson & Johnson – Employee. Johnson & Johnson – Employee. Johnson & Johnson – Stock Options, Stock-publicly held company(excluding mutual/index funds).
Silke Jorgens: Johnson & Johnson – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Hongyan Zhang: Johnson & Johnson – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Lingjing Jiang: Johnson & Johnson – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Tadakazu Hisamatsu: AbbVie GK – Consultant, Grant/Research Support, Lecture fees. Boston Scientific Corporation – Grant/Research Support. Bristol Myers Squibb – Consultant. Daiichi Sankyo Co. Ltd. – Grant/Research Support, Honararium. EA Pharma Co. Ltd. – Consultant, Grant/Research Support, Lecture fees. Gilead Sciences – Consultant. JIMRO Co. Ltd. – Grant/Research Support, Lecture fees. Johnson & Johnson – Consultant, Lecture fees. Kissei Pharmaceutical Co. Ltd. – Grant/Research Support, Lecture fees. Kyorin Pharmaceutical Co. Ltd. – Grant/Research Support, Lecture fees. Lilly – Consultant. Mitsubishi Tanabe Pharma Corporation – Consultant, Grant/Research Support, Lecture fees. Mochida Pharmaceutical Co. Ltd. – Grant/Research Support, Lecture fees. Nichi-Iko Pharmaceutical Co. Ltd – Consultant. Nippon Kayaku Co. Ltd. – Grant/Research Support. Pfizer Inc. – Consultant, Grant/Research Support, Lecture fees. Takeda Pharmaceutical Co. Ltd. – Grant/Research Support, Lecture fees. Zeria Pharmaceutical Co – Grant/Research Support.
David Rubin: AbbVie – Advisory Committee/Board Member, Consultant, Speakers Bureau. Altrubio – Advisory Committee/Board Member, Consultant, Speakers Bureau, Stock Options. Apex – Advisory Committee/Board Member, Consultant, Speakers Bureau. Avalo – Advisory Committee/Board Member, Consultant, Speakers Bureau. Bristol Myers Squibb – Advisory Committee/Board Member, Consultant, Speakers Bureau. Buhlmann Diagnostics – Advisory Committee/Board Member, Consultant, Speakers Bureau. Celgene – Advisory Committee/Board Member, Consultant, Speakers Bureau. Connect BioPharma – Advisory Committee/Board Member, Consultant, Speakers Bureau. Cornerstones Health, Inc. – Advisory Committee/Board Member. Crohn's & Colitis Foundation – Advisory Committee/Board Member. Datos Health – Stock Options. Intouch Group – Advisory Committee/Board Member, Consultant, Speakers Bureau. Iterative Health – Advisory Committee/Board Member, Consultant, Speakers Bureau, Stock Options. Johnson & Johnson – Advisory Committee/Board Member, Consultant, Speakers Bureau. Lilly – Advisory Committee/Board Member, Consultant, Speakers Bureau. Pfizer – Advisory Committee/Board Member, Consultant, Speakers Bureau. Samsung Neurologica – Advisory Committee/Board Member, Consultant, Speakers Bureau. Takeda – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau.
Silvio Danese: AbbVie – Consultant, Lecture fees. Alimentiv – Consultant. Allergan – Consultant. Amgen – Consultant, Lecture fees. AstraZeneca – Consultant. Athos Therapeutics – Consultant. Biogen – Consultant. Boehringer Ingelheim – Consultant. Celgene – Consultant. Celltrion – Consultant. Eli Lilly – Consultant. Enthera – Consultant. F. Hoffmann-La Roche Ltd – Consultant. Ferring Pharmaceuticals Inc. – Consultant, Lecture fees. Gilead – Consultant, Lecture fees. Hospira – Consultant. Inotrem – Consultant. Johnson & Johnson – Consultant, Lecture fees. MSD – Consultant. Mundipharma – Consultant. Mylan – Consultant, Lecture fees. Pfizer – Consultant, Lecture fees. Sandoz – Consultant. Sublimity Therapeutics – Consultant. Takeda – Consultant, Lecture fees. TiGenix – Consultant. UCB Inc. – Consultant. Vifor (International) Ltd. – Consultant.
Jessica R.. Allegretti, MD, MPH, FACG1, Laurent Peyrin-Biroulet, MD, PhD2, Millie D. Long, MD, FACG3, Matthew Germinaro, MD4, Thomas Baker, MD4, Mary Kavalam, 4, Yelina Alvarez, 4, Karen Hertzog, 4, Silke Jorgens, 5, Hongyan Zhang, 4, Lingjing Jiang, 4, Tadakazu Hisamatsu, MD, PhD6, David T. Rubin, MD7, Silvio Danese, MD, PhD8, 4, Efficacy and Safety of Subcutaneous Guselkumab Induction and Maintenance Therapy in Participants With Ulcerative Colitis: Results Through Week 48 From the Phase 3 ASTRO Study, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.



