Oral Paper Presentation
Annual Scientific Meeting
Session: Plenary Session 4B: IBD
72 - Real World Outcomes of Upadacitinib Compared to Risankizumab as Second-Line Therapy in Patients With Crohn’s Disease Exposed to Tumor Necrosis Factor-Alpha Inhibitors
Wednesday, October 29, 2025
9:40 AM - 9:50 AM PDT
Location: North Ballroom 120BC

Mira Sridharan, MD (she/her/hers)
Boston Medical Center
Boston, MA
Presenting Author(s)
Mira Sridharan, MD1, Nikita Patel, MD1, Gursimran Kochhar, MD2, Aakash Desai, MD3
1Boston Medical Center, Boston, MA; 2Division of Gastroenterology, Hepatology, and Nutrition, Allegheny Health Network, Pittsburgh, PA; 3Allegheny Health Network, Pittsburgh, PA
Introduction: Upadacitinib (Upa) and risankizumab (Risa) are effective therapies for moderate-severe Crohn’s disease (CD) however, there is limited real-world data comparing their outcomes in patients exposed to a tumor necrosis factor-alpha inhibitor (TNFi).
Methods: A retrospective cohort study was conducted with TriNetX database in adults with CD who initiated either Upa or Risa between 6/1/2023 and 9/30/2024 as second-line therapy following exposure to a TNFi. Patients who were not on a TNFi or those underwent surgery in the preceding 1 year prior were excluded. Primary aim was to assess the risk of a composite outcome of corticosteroids and/or surgery after 30 days and within 12 months. Secondary outcomes included risk of steroid use, surgery, change in therapy, fecal calprotectin < 200 ug/g, CRP ≥10 mg/L, all-cause hospitalization and emergency department (ED) visits. 1:1 propensity score matching (PSM) was performed between the two cohorts based on demographics, comorbidities, disease location, phenotype, laboratory values, type of TNFi, and history of surgery/steroid use. Risk was quantified as adjusted odds ratio (aOR) with 95% confidence intervals (CIs).
Results: 257 patients were in the Upa cohort (mean age 39.1 ± 15.8 years; 51.3% male; 13.2% with prior exposure to ≥2TNFi), and 568 in the Risa cohort (mean age 40.7 ± 16.1 years; 48.4% male; 13.02% with prior exposure to ≥2TNFi). There was a higher proportion of patients with previous surgery (27.4% vs 18.2%, p=0.004) and stricturing disease (14.7% vs 8.5%, p=0.01) in the Risa cohort before PSM. After PSM, no significant difference was observed in the primary composite outcome of steroid use and/or surgery (aOR 0.89, 95% CI 0.60-1.30) between the Upa and Risa cohorts. Patients on Upa had significantly lower risk of IV steroid use independently (aOR 0.61; 95% CI 0.37–0.98), but a significantly higher risk of therapy change (aOR 1.88; 95% CI, 1.15–3.08). No significant differences were observed with respect to risk of surgery(OR 1.59, 95% CI 0.80-3.14), oral steroid use (OR 1.25; 95% CI, 0.82–1.91), hospitalization (OR 1.13; 95% CI, 0.69–1.85), fecal calprotectin < 200 µg/g (OR 0.97; 95% CI, 0.46–2.03) and CRP ≥ 10 mg/L (OR 1.39, 95% CI 0.79-2.42). The risk of adverse events was low in both cohorts.
Discussion: Our study utilizing real-world data suggests that Upa and Risa are both effective second line therapies in TNFi-exposed patients with CD however, patients on Upa were more likely to experience change in therapy.
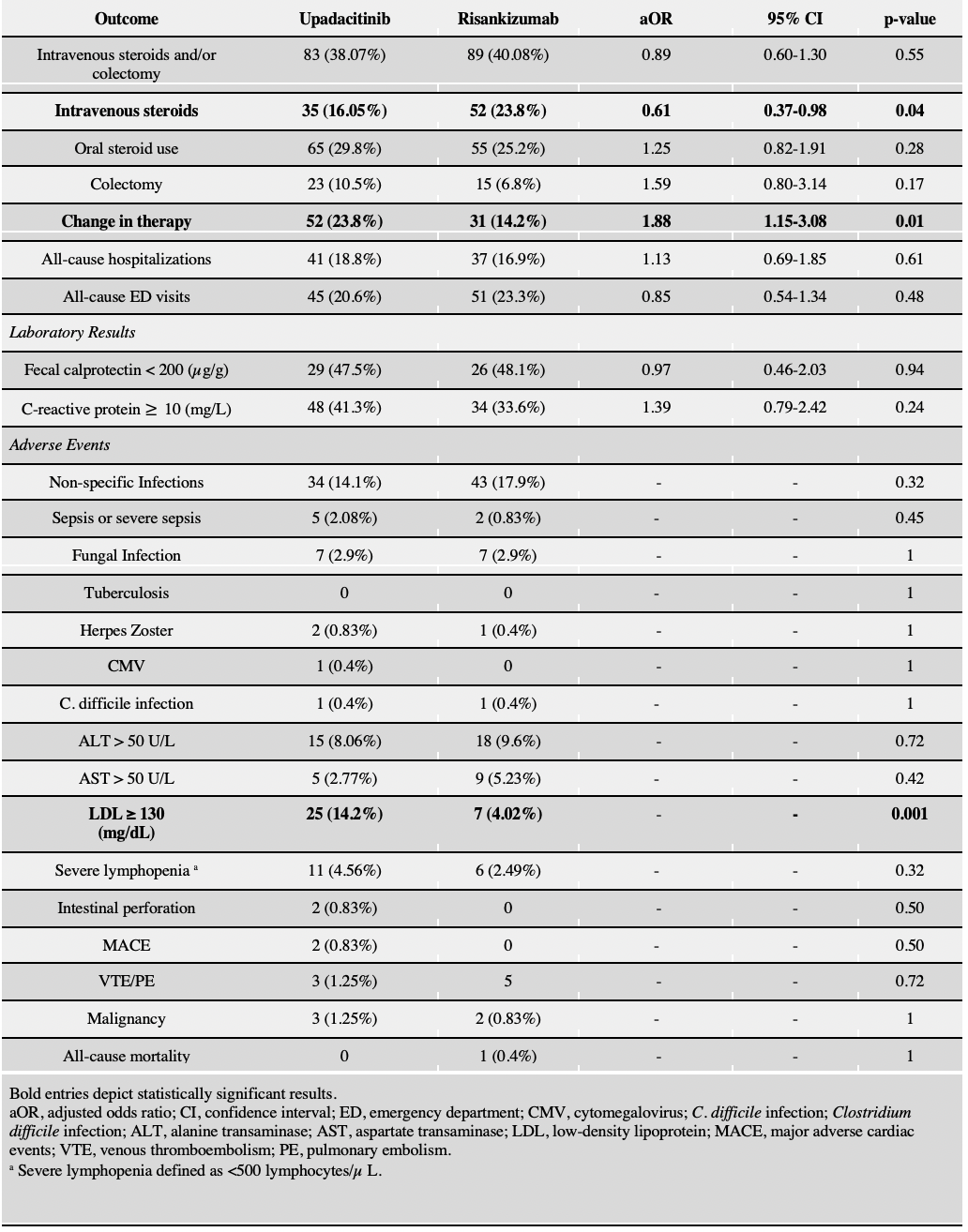
Figure: Table 1: Comparison of outcomes and adverse effects between the Upadacitinib cohort and Risankizumab cohort within 12 months of initiation after 1:1 propensity score matching.
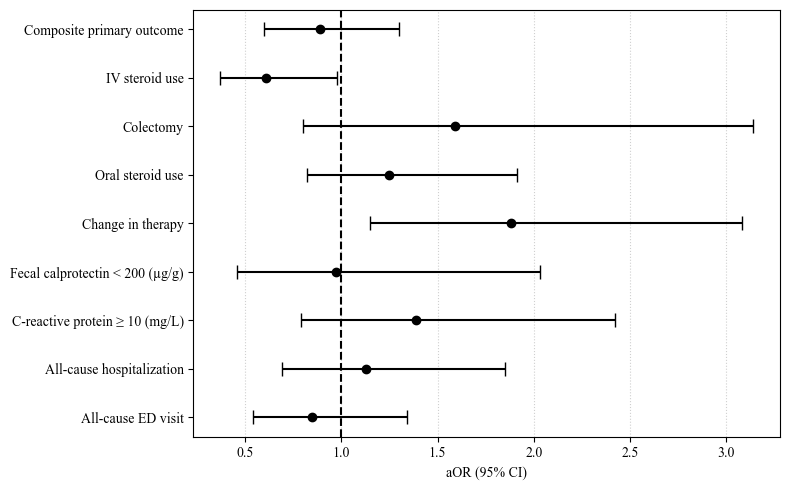
Figure: Figure 1: Forest Plot of aOR with 95% CI comparing clinical outcomes between the Upadacitinib and Risankizumab cohorts after propensity score matching. aOR, adjusted odds ratio; CI, confidence intervals; IV, intravenous; ED, emergency department.
Disclosures:
Mira Sridharan indicated no relevant financial relationships.
Nikita Patel indicated no relevant financial relationships.
Gursimran Kochhar: Boston Scientific Endoscopy – Consultant. Corvetas Research Foundation – Advisory Committee/Board Member. Digbi Health – Stock Options. Eli Lilly and Company – Advisory Committee/Board Member, Speakers Bureau. Exact Sciences – Consultant. GIE Medical – Advisory Committee/Board Member. Olympus Endoscopy – Consultant. Pentax Endoscopy – Consultant. Pharmacosmos – Advisory Committee/Board Member. Takeda – Consultant.
Aakash Desai indicated no relevant financial relationships.
Mira Sridharan, MD1, Nikita Patel, MD1, Gursimran Kochhar, MD2, Aakash Desai, MD3, 72, Real World Outcomes of Upadacitinib Compared to Risankizumab as Second-Line Therapy in Patients With Crohn’s Disease Exposed to Tumor Necrosis Factor-Alpha Inhibitors, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Boston Medical Center, Boston, MA; 2Division of Gastroenterology, Hepatology, and Nutrition, Allegheny Health Network, Pittsburgh, PA; 3Allegheny Health Network, Pittsburgh, PA
Introduction: Upadacitinib (Upa) and risankizumab (Risa) are effective therapies for moderate-severe Crohn’s disease (CD) however, there is limited real-world data comparing their outcomes in patients exposed to a tumor necrosis factor-alpha inhibitor (TNFi).
Methods: A retrospective cohort study was conducted with TriNetX database in adults with CD who initiated either Upa or Risa between 6/1/2023 and 9/30/2024 as second-line therapy following exposure to a TNFi. Patients who were not on a TNFi or those underwent surgery in the preceding 1 year prior were excluded. Primary aim was to assess the risk of a composite outcome of corticosteroids and/or surgery after 30 days and within 12 months. Secondary outcomes included risk of steroid use, surgery, change in therapy, fecal calprotectin < 200 ug/g, CRP ≥10 mg/L, all-cause hospitalization and emergency department (ED) visits. 1:1 propensity score matching (PSM) was performed between the two cohorts based on demographics, comorbidities, disease location, phenotype, laboratory values, type of TNFi, and history of surgery/steroid use. Risk was quantified as adjusted odds ratio (aOR) with 95% confidence intervals (CIs).
Results: 257 patients were in the Upa cohort (mean age 39.1 ± 15.8 years; 51.3% male; 13.2% with prior exposure to ≥2TNFi), and 568 in the Risa cohort (mean age 40.7 ± 16.1 years; 48.4% male; 13.02% with prior exposure to ≥2TNFi). There was a higher proportion of patients with previous surgery (27.4% vs 18.2%, p=0.004) and stricturing disease (14.7% vs 8.5%, p=0.01) in the Risa cohort before PSM. After PSM, no significant difference was observed in the primary composite outcome of steroid use and/or surgery (aOR 0.89, 95% CI 0.60-1.30) between the Upa and Risa cohorts. Patients on Upa had significantly lower risk of IV steroid use independently (aOR 0.61; 95% CI 0.37–0.98), but a significantly higher risk of therapy change (aOR 1.88; 95% CI, 1.15–3.08). No significant differences were observed with respect to risk of surgery(OR 1.59, 95% CI 0.80-3.14), oral steroid use (OR 1.25; 95% CI, 0.82–1.91), hospitalization (OR 1.13; 95% CI, 0.69–1.85), fecal calprotectin < 200 µg/g (OR 0.97; 95% CI, 0.46–2.03) and CRP ≥ 10 mg/L (OR 1.39, 95% CI 0.79-2.42). The risk of adverse events was low in both cohorts.
Discussion: Our study utilizing real-world data suggests that Upa and Risa are both effective second line therapies in TNFi-exposed patients with CD however, patients on Upa were more likely to experience change in therapy.

Figure: Table 1: Comparison of outcomes and adverse effects between the Upadacitinib cohort and Risankizumab cohort within 12 months of initiation after 1:1 propensity score matching.

Figure: Figure 1: Forest Plot of aOR with 95% CI comparing clinical outcomes between the Upadacitinib and Risankizumab cohorts after propensity score matching. aOR, adjusted odds ratio; CI, confidence intervals; IV, intravenous; ED, emergency department.
Disclosures:
Mira Sridharan indicated no relevant financial relationships.
Nikita Patel indicated no relevant financial relationships.
Gursimran Kochhar: Boston Scientific Endoscopy – Consultant. Corvetas Research Foundation – Advisory Committee/Board Member. Digbi Health – Stock Options. Eli Lilly and Company – Advisory Committee/Board Member, Speakers Bureau. Exact Sciences – Consultant. GIE Medical – Advisory Committee/Board Member. Olympus Endoscopy – Consultant. Pentax Endoscopy – Consultant. Pharmacosmos – Advisory Committee/Board Member. Takeda – Consultant.
Aakash Desai indicated no relevant financial relationships.
Mira Sridharan, MD1, Nikita Patel, MD1, Gursimran Kochhar, MD2, Aakash Desai, MD3, 72, Real World Outcomes of Upadacitinib Compared to Risankizumab as Second-Line Therapy in Patients With Crohn’s Disease Exposed to Tumor Necrosis Factor-Alpha Inhibitors, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.


