Oral Paper Presentation
Annual Scientific Meeting
Session: Plenary Session 4B: IBD
73 - Efficacy and Safety of Obefazimod in Patients With Moderately to Severely Active Ulcerative Colitis: Results From Two, Phase 3, Randomised, Double-Blind, Placebo-Controlled, 8-Week Induction Trials (ABTECT 1 & 2) (Late-Breaking Abstract)
Wednesday, October 29, 2025
9:50 AM - 10:00 AM PDT
Location: North Ballroom 120BC
.jpeg.jpg)
Bruce E. Sands, MD, MS, FACG
Chief, Division of Gastroenterology
Icahn School of Medicine at Mount Sinai
New York, NY
Late Breaking Abstract Presenter(s)
Bruce E. Sands, MD, FACG1, Silvio Danese, MD2, Laurent Peyrin-Biroulet, MD3, Marla C. Dubinsky, MD4, Tadakazu Hisamatsu, MD5, Herbert Tilg, MD6, Raja Atreya, MD7, Alessandro Armuzzi, MD8, Xavier Treton, MD9, Filip Baert, MD10, Ursula Seidler, MD11, Fabio Cataldi, MD12, Doug Jacobstein, MD12, Christopher J. Rabbat, PhD12, Kejia Shan, MS12, George Aaron Duvall, MD13, Britta Siegmund, MD14, Parambir S. Dulai, MD15, David T. Rubin, MD, FACG16, Séverine Vermeire, MD171Icahn School of Medicine at Mount Sinai, New York, NY; 2Gastroenterology and Endoscopy IRCCS Ospedale San Raffaele, Italy, Milan, Lombardia, Italy; 3INFINY Institute, INSERM NGERE, CHRU Nancy, Vandœuvre-lès-Nancy, Lorraine, France; 4Mount Sinai Kravis Children’s Hospital, New York, USA, New York, NY; 5 Kyorin University School of Medicine, Tokyo, Tokyo, Japan; 6 University Innsbruck, Innsbruck, Tirol, Austria; 7University Hospital Erlangen, Erlangen, Bayern, Germany; 8IRCCS Humanitas Research Hospital, Milan, Lombardia, Italy; 9Groupe Hospitalier Prive Ambroise Pare – Hartmann, Institut des MICI, Neuilly sur Seine, Ile-de-France, France; 10AZ Delta, Roeselare, West-Vlaanderen, Belgium; 11Medizinische Hochschule Hannover, Hannover, Sachsen, Germany; 12Abivax, Paris, Ile-de-France, France; 13Tyler Research Institute, LLC, Tyler, TX; 14Charité Universitätsmedizin Berlin, Berlin, Germany; 15Feinberg School of Medicine Northwestern University - Chicago, IL; 16University of Chicago Medicine, Chicago, IL; 17University Hospitals Leuven, Leuven, Oost-Vlaanderen, Belgium
Introduction: Obefazimod (Obe), an oral, once-daily (QD), small molecule which enhances expression of microRNA-124 was studied in patients (pts) with moderately to severely active ulcerative colitis (UC) in Phase 2 induction trials and in subsequent open-label maintenance studies1. Here we report the efficacy and safety of two Phase 3, 8-week induction trials in adult pts with UC from ABTECT-1 [NCT05507203] and ABTECT-2 [NCT05507216].
Methods: The randomized, double-blind, placebo-controlled ABTECT trials enrolled pts with UC (MMS ≥ 5 with RBS ≥ 1 and centrally read endoscopic score ≥2) who had inadequate response, loss of response, or intolerance to at least one prior therapy (with no upper limit), including corticosteroids, immunosuppressants, biologics, S1P receptor modulators and/or JAK inhibitors. Pts were randomized 2:1:1 to Obe 50 mg QD (Obe-50), Obe 25 mg QD (Obe-25) or placebo (PBO) for 8 weeks. The primary endpoint was clinical remission (per MMS) and key secondary endpoints included clinical response, endoscopic improvement, and histo-endoscopic mucosal improvement.
Results: 1272 pts were randomized and treated in ABTECT-1 (636) and ABTECT-2 (636). In both trials, baseline demographics and disease characteristics were similar between groups; 45.3% and 49.3% of pts had inadequate response to ≥ 1 advanced therapy. In both trials, a significantly higher proportion of pts receiving Obe-50 achieved clinical remission and all key secondary endpoints vs. PBO (Figure). In ABTECT-1, but not ABTECT-2, a significantly higher proportion of pts receiving Obe-25 achieved clinical remission and all key secondary endpoints. In a pooled analysis, both Obe-50 and Obe-25 met all primary and secondary endpoints with nominal significance (Figure). The rate of serious adverse events and treatment emergent adverse events (TEAEs) leading to study drug discontinuation for Obe-treated pts were similar to PBO. Proportions of pts who reported at least one TEAE were higher for Obe-50 and similar for Obe-25 vs. PBO in both trials. Headache was the most frequent TEAE; they were mild, short in duration, and not a barrier to treatment as evidenced by a low discontinuation rate (Table).
Discussion: In both ABTECT trials, Obe treatment led to statistically significant improvements in clinical, endoscopic, symptomatic, and combined endoscopic-histologic endpoints at week 8. Obe was well tolerated and the overall safety profile was similar to previous studies, with no new safety signals.
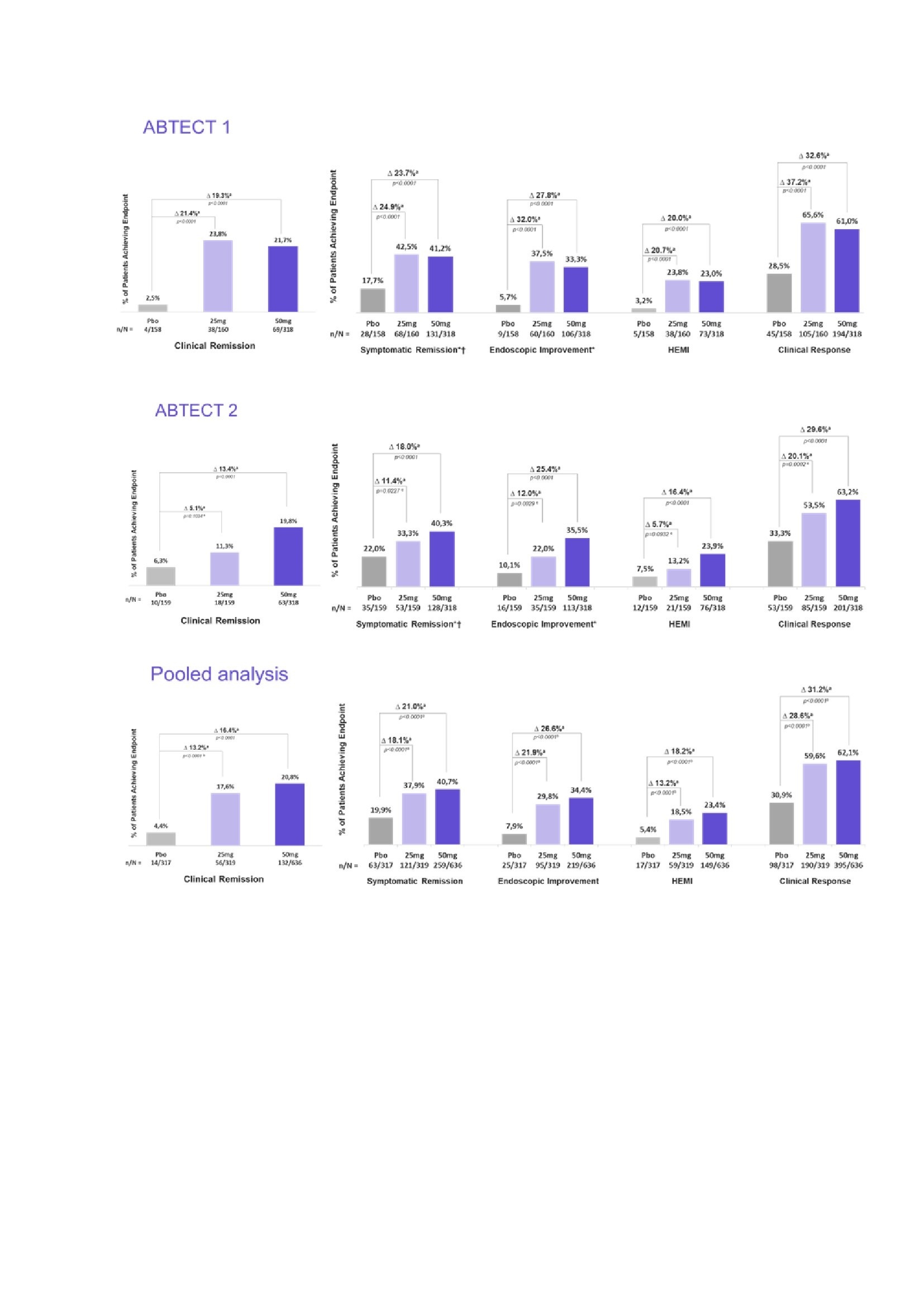
Figure: Efficacy results at week 8 - Primary and secondary endpoints, ABTECT-1 and -2, pooled analysis‡ [a] % Difference is for obefazimod minus placebo and is based on estimated common risk difference using the Mantel-Haenszel weights adjusting for the randomization stratification factors: inadequate response to advanced therapies (yes/no), Baseline oral corticosteroids usage (yes/no), and region (Japan/rest of world) [ABTECT-2 only]. P-values are two sided. [b] Because this is a pooled analysis, all p-values are nominal. [c] 25mg did not meet the primary endpoint at week 8 in ABTECT-2 in the FDA testing protocol, therefore p-values for key secondary endpoints for the 25mg arm in ABTECT 2 are nominal. NRI is used for subjects with missing outcomes at Week 8 and subjects reporting any IE prior to Week 8. Clinical remission is defined as SFS = 0 or 1, and RBS = 0 and MES = 0 or 1 (MES of 1 modified to exclude friability); Endoscopic improvement is defined as MES = 0 or 1 (MES of 1 modified to exclude friability). Clinical response is defined as a reduction from Baseline in MMS >= 2 points and a relative reduction from Baseline in MMS >= 30%, and a reduction from Baseline in RBS >= 1 point and/or RBS = 0 or 1. HEMI is defined as MES = 0 or 1 and Geboes Index score <3.1 Symptomatic remission is defined as RBS=0 and SFS= 0 or 1 †For FDA testing protocol, symptomatic remission was an “other secondary” endpoint, not multiplicity controlled * Endoscopic improvement/symptomatic remission were co-primary endpoints for the EMA protocol and were met by both doses in both trials ‡Hierarchical testing strategy was used starting with 50mg for the primary endpoint followed by the key secondary endpoints; the 25mg was subsequently tested for the primary endpoint followed by the key secondary endpoints.
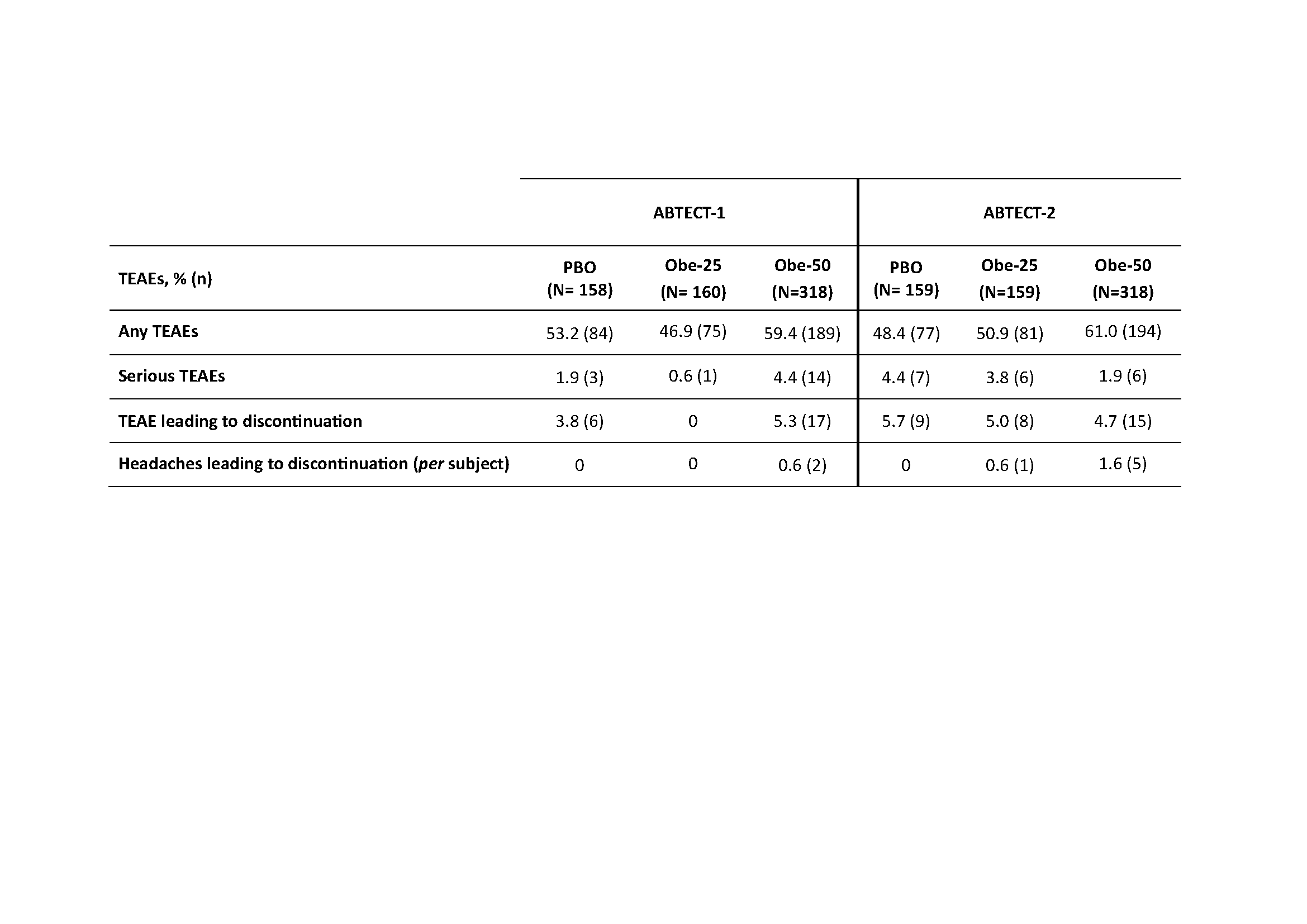
Table: Treatment-emergent adverse events (TEAEs) throughout 8 weeks in ABTECT-1 and ABTECT-2 Phase 3 trials
Disclosures:
Bruce Sands: AbbVie; Consultant. Abivax; Consultant. Adiso Therapeutics; Consultant. AgomAb; Consultant. Alimentiv; Consultant. Amgen; Consultant. AnaptysBio; Consultant. Arena; Consultant. Artugen Therapeutics; Consultant. AstraZeneca; Consultant. Biolojic Design; Consultant. Biora Therapeutics; Consultant. Boehringer Ingelheim; Consultant. Bristol Meyer Squibb; Consultant. Celltrion; Consultant. Enthera; Consultant. Enveda Biosciences; Consultant. Equillium; Consultant. Evommune; Consultant. Ferring; Consultant. Fzata; Consultant. Galapagos; Consultant. Genentech-Roche; Consultant. Gilead; Consultant. Gossamer Bio; Consultant. GSK; Consultant. Imhotex; Consultant. Index Pharmaceuticals; Consultant. Innovation Pharmaceuticals; Consultant. Janssen; Consultant. Kaleido; Consultant. Kallyope; Consultant. Lilly; Consultant. Merck; Consultant. Microba; Consultant. Microbiotica; Consultant. Mitsubishi Tanabe; Consultant. Mobius Care; Consultant. Morphic Therapeutics; Consultant. MRM Health; Consultant. Nexus Therapeutics; Consultant. Nimbus Discovery; Consultant. Odyssey Therapeutics; Consultant. OSE Immunotherapeutics; Consultant. Palisade Bio; Consultant. Pfizer; Consultant. Protagonist Therapeutics; Consultant. Q32 Bio; Consultant. Rasayana Therapeutics; Consultant. Recludix Therapeutics; Consultant. Reistone Biopharma; Consultant. Sanofi; Consultant. Sorriso Therapeutics; Consultant. Spyre Therapeutics; Consultant. Takeda; Consultant. Target RWE; Consultant. Teva; Consultant. TLL Pharmaceutical; Consultant. Tr1X; Consultant. Trex Bio; Consultant. Union Therapeutics; Consultant. Ventyx Biosciences; Consultant.
Silvio Danese: AbbVie; Consultant. Allergan; Consultant. Biogen; Consultant. Boehringer Ingelheim; Consultant. Celgene; Consultant. Celltrion; Consultant. Ferring; Consultant. Hospira; Consultant. Johnson & Johnson; Consultant. Merck; Consultant. MSD; Consultant. Mundipharma; Consultant. Pfizer Inc; Consultant. Sandoz; Consultant. Takeda; Consultant. Tigenix; Consultant. UCB Pharma; Consultant. Vifor; Consultant.
Laurent Peyrin-Biroulet: AbbVie; Consultant. Abivax; Consultant. Adacyte; Consultant. Alimentiv; Consultant. Amgen; Consultant. Applied Molecular Transport; Consultant. Arena; Consultant. Banook; Consultant. Biogen; Consultant. BMS; Consultant. Celltrion; Consultant, Grant/Research Support. Connect Biopharm; Consultant. Cytoki Pharma; Consultant. Enthera; Consultant. Ferring; Consultant. Fresenius Kabi; Consultant, Grant/Research Support. Galapagos; Consultant. Genentech; Consultant. Gossamer Bio; Consultant. GSK; Consultant. IAC Image Analysis; Consultant. Index Pharmaceuticals; Consultant. Inotrem; Consultant. Janssen; Consultant. Lilly; Consultant. Medac; Consultant, Grant/Research Support. Mopac; Consultant. Morphic; Consultant. MSD; Consultant, Grant/Research Support. Nordic Pharma; Consultant. Novartis; Consultant. Oncodesign; Consultant. ONO Pharma; Consultant. OSE Immunotherapeutics; Consultant. Pandion Therapeuthics; Consultant. Par' Immune; Consultant. Pfizer; Consultant. Prometheus; Consultant. Protagonist; Consultant. Roche; Consultant. Samsung; Consultant. Sandoz; Consultant. Sanofi; Consultant. Satisfay; Consultant. Takeda; Consultant, Grant/Research Support. Telavant; Consultant. Theravance; Consultant. Thermo Fischer; Consultant. Tigenix; Consultant. Tillots; Consultant. Vectivbio; Consultant. Ventyx; Consultant. Viatris; Consultant. Ysopia; Consultant.
Marla Dubinsky: AbbVie; Consultant. Abivax; Consultant. Arena Pharmaceuticals; Consultant. AstraZeneca; Consultant. Boehringer Ingelheim; Consultant. Bristol Meyer Squibb; Consultant. F. Hoffmann-La Roche Ltd; Consultant. Genentech; Consultant. Gilead; Consultant. Janssen; Consultant. Lilly; Consultant. Merck; Consultant. Pfizer Inc; Consultant. Prometheus; Consultant. Takeda; Consultant.
Tadakazu Hisamatsu: AbbVie; Consultant, Grant/Research Support. Abivax; Consultant. Boston Scientific Corporation; Grant/Research Support. Bristol Meyer Squibb; Consultant. EA pharma; Consultant, Grant/Research Support. Gilead; Consultant. Janssen; Consultant. JIMRO Co. Ltd; Grant/Research Support. Kissei Pharmaceutical; Grant/Research Support, Lecture fee. Kyorin Pharmaceutical; Grant/Research Support. Lilly; Consultant. Mitsubishi Tanabe; Consultant, Grant/Research Support. Mochida Pharmaceutical; Grant/Research Support. Nippon Kayaku; Grant/Research Support. Pfizer Inc; Consultant, Grant/Research Support. Takeda; Grant/Research Support. Zeria Pharmaceutical Co. Ltd; Grant/Research Support.
Herbert Tilg: AbbVie; Consultant. Abivax; Consultant. Dr Falk Pharma; Consultant. Ferring; Consultant. Galapagos; Consultant. Microbiotica; Consultant. MSD; Consultant. Pfizer; Consultant. Takeda; Consultant.
Raja Atreya: AbbVie; Consultant. Abivax; Consultant. AstraZeneca; Consultant. Bristol Meyer Squibb; Consultant. Celltrion; Consultant. Galapagos; Consultant. Johnson & Johnson; Consultant. Lilly; Consultant. MSD; Consultant. Pfizer; Consultant. Takeda; Consultant.
Alessandro Armuzzi: AbbVie; Consultant. Abivax; Consultant. Alpha Sigma; Consultant. AstraZeneca; Consultant. Biogen; Consultant, Grant/Research Support. Boehringer Ingelheim; Consultant. Bristol Meyer Squibb; Consultant. Celltrion; Consultant. Enthera; Consultant. Ferring; Consultant. Galapagos; Consultant. Gilead; Consultant. Giuliani; Consultant. Janssen; Consultant. Lilly; Consultant. Lionhealth; Consultant. MSD; Consultant, Grant/Research Support. Nestlé; Consultant. Pfizer; Consultant, Grant/Research Support. Protagonist; Consultant. Roche; Consultant. Samsung Bioepis; Consultant. Sandoz; Consultant. Sanofi; Consultant. Takeda; Consultant, Grant/Research Support. Teva; Consultant. Tillots; Consultant.
Xavier Treton: AbbVie; Consultant. Abivax; Consultant. Alpha Sigma; Consultant. Biogen; Consultant. Celltrion; Consultant. Dr Falk Pharma; Consultant. Fresenius Kabi; Consultant. Johnson & Johnson; Consultant. Lilly; Consultant. MSD; Consultant. Pfizer; Consultant. Takeda; Consultant. Thabor Therapeutics; Consultant. Tillots; Consultant.
Filip Baert: AbbVie; Consultant, Grant/Research Support. Amgen; Consultant, Grant/Research Support. Arena Pharmaceuticals; Consultant. Celgene; Consultant. Celltrion; Consultant. Eurogenerics; Grant/Research Support. Ferring; Consultant. Fresenius Kabi; Consultant. Johnson & Johnson; Consultant, Grant/Research Support. MSD; Consultant. Pfizer Inc; Consultant. Sandoz; Consultant. Takeda; Grant/Research Support.
Ursula Seidler: AbbVie; Consultant, Grant/Research Support. Abivax; Consultant, Grant/Research Support. Amgen; Consultant. Boehringer Ingelheim; Grant/Research Support. Bristol Meyer Squibb; Grant/Research Support. Celgene; Grant/Research Support. Galapagos; Consultant, Grant/Research Support. Gilead; Grant/Research Support. Janssen; Consultant, Grant/Research Support. Lilly; Consultant, Grant/Research Support. Pfizer; Grant/Research Support. Roche; Grant/Research Support. Takeda; Grant/Research Support.
Fabio Cataldi: Abivax; Employee.
Doug Jacobstein: Abivax; Employee.
Christopher J. Rabbat: Abivax; Employee.
Kejia Shan: Abivax; Employee.
George Aaron Duvall indicated no relevant financial relationships.
Britta Siegmund: AbbVie; Consultant. Abivax; Consultant. Boehringer Ingelheim; Consultant. Bristol Meyer Squibb; Consultant. Dr Falk Pharma; Consultant. Endpoint Health; Consultant. Falk; Consultant. Galapagos; Consultant. Gilead; Consultant. Janssen; Consultant. Landos; Consultant. Lilly; Consultant. Materia Prima; Consultant. Pfizer; Consultant, Grant/Research Support. Predictimmune; Consultant. Takeda; Consultant.
Parambir Dulai: AbbVie; Consultant. Abivax; Consultant. Adiso; Consultant. Alimentiv; Consultant. Bristol Meyer Squibb; Consultant. Celltrion; Consultant. Genentech; Consultant. Geneoscopy; Consultant. Janssen; Consultant. Pfizer; Consultant. Takeda; Consultant.
David Rubin: AbbVie; Consultant. Abivax; Consultant. Altrubio; Consultant. Athos Therapeutics; Consultant. Bristol Meyer Squibb; Consultant. Celltrion; Consultant. Connect Biopharma; Consultant. Genentech; Consultant. Iterative Health; Consultant. Janssen; Consultant. Johnson & Johnson; Consultant. Lilly; Consultant. Merck; Consultant. Odyssey Therapeutics; Consultant. Pfizer; Consultant. Sanofi; Consultant. Spyre; Consultant. Takeda; Consultant, Grant/Research Support. Vedanta Biosciences; Consultant. Ventyx Biosciences; Consultant.
Séverine Vermeire: AbbVie; Consultant, Grant/Research Support. Abivax; Consultant. AbolerIS Pharma; Consultant. AgomAb; Consultant. Alimentiv; Consultant. Arena; Consultant. AstraZeneca; Consultant. Avaxia; Consultant. BMS; Consultant. Boehringer Ingelheim; Consultant. Celgene; Consultant. CVasThera; Consultant. Cytoki Pharma; Consultant. Dr Falk Pharma; Consultant. Ferring; Consultant. Galapagos; Consultant, Grant/Research Support. Genentech-Roche; Consultant. Gilead; Consultant. GSK; Consultant. Hospira; Consultant. Imidomics; Consultant. Janssen; Consultant. Johnson & Johnson; Consultant, Grant/Research Support. Lilly; Consultant. Materia Prima; Consultant. MiroBio; Consultant. Morphic; Consultant. MRM Health; Consultant. MSD; Consultant. Mundipharma; Consultant. Pfizer; Consultant, Grant/Research Support. Prodigest; Consultant. Progenity; Consultant. Prometheus; Consultant. Robarts Clinical Trials; Consultant. Second Genome; Consultant. Shire; Consultant. Surrozen; Consultant. Takeda; Consultant, Grant/Research Support. Theravance; Consultant. Tillots; Consultant. Zealand Pharma; Consultant.
Introduction: Obefazimod (Obe), an oral, once-daily (QD), small molecule which enhances expression of microRNA-124 was studied in patients (pts) with moderately to severely active ulcerative colitis (UC) in Phase 2 induction trials and in subsequent open-label maintenance studies1. Here we report the efficacy and safety of two Phase 3, 8-week induction trials in adult pts with UC from ABTECT-1 [NCT05507203] and ABTECT-2 [NCT05507216].
Methods: The randomized, double-blind, placebo-controlled ABTECT trials enrolled pts with UC (MMS ≥ 5 with RBS ≥ 1 and centrally read endoscopic score ≥2) who had inadequate response, loss of response, or intolerance to at least one prior therapy (with no upper limit), including corticosteroids, immunosuppressants, biologics, S1P receptor modulators and/or JAK inhibitors. Pts were randomized 2:1:1 to Obe 50 mg QD (Obe-50), Obe 25 mg QD (Obe-25) or placebo (PBO) for 8 weeks. The primary endpoint was clinical remission (per MMS) and key secondary endpoints included clinical response, endoscopic improvement, and histo-endoscopic mucosal improvement.
Results: 1272 pts were randomized and treated in ABTECT-1 (636) and ABTECT-2 (636). In both trials, baseline demographics and disease characteristics were similar between groups; 45.3% and 49.3% of pts had inadequate response to ≥ 1 advanced therapy. In both trials, a significantly higher proportion of pts receiving Obe-50 achieved clinical remission and all key secondary endpoints vs. PBO (Figure). In ABTECT-1, but not ABTECT-2, a significantly higher proportion of pts receiving Obe-25 achieved clinical remission and all key secondary endpoints. In a pooled analysis, both Obe-50 and Obe-25 met all primary and secondary endpoints with nominal significance (Figure). The rate of serious adverse events and treatment emergent adverse events (TEAEs) leading to study drug discontinuation for Obe-treated pts were similar to PBO. Proportions of pts who reported at least one TEAE were higher for Obe-50 and similar for Obe-25 vs. PBO in both trials. Headache was the most frequent TEAE; they were mild, short in duration, and not a barrier to treatment as evidenced by a low discontinuation rate (Table).
Discussion: In both ABTECT trials, Obe treatment led to statistically significant improvements in clinical, endoscopic, symptomatic, and combined endoscopic-histologic endpoints at week 8. Obe was well tolerated and the overall safety profile was similar to previous studies, with no new safety signals.
- Vermeire et al JCC 2023, 1689-97

Figure: Efficacy results at week 8 - Primary and secondary endpoints, ABTECT-1 and -2, pooled analysis‡ [a] % Difference is for obefazimod minus placebo and is based on estimated common risk difference using the Mantel-Haenszel weights adjusting for the randomization stratification factors: inadequate response to advanced therapies (yes/no), Baseline oral corticosteroids usage (yes/no), and region (Japan/rest of world) [ABTECT-2 only]. P-values are two sided. [b] Because this is a pooled analysis, all p-values are nominal. [c] 25mg did not meet the primary endpoint at week 8 in ABTECT-2 in the FDA testing protocol, therefore p-values for key secondary endpoints for the 25mg arm in ABTECT 2 are nominal. NRI is used for subjects with missing outcomes at Week 8 and subjects reporting any IE prior to Week 8. Clinical remission is defined as SFS = 0 or 1, and RBS = 0 and MES = 0 or 1 (MES of 1 modified to exclude friability); Endoscopic improvement is defined as MES = 0 or 1 (MES of 1 modified to exclude friability). Clinical response is defined as a reduction from Baseline in MMS >= 2 points and a relative reduction from Baseline in MMS >= 30%, and a reduction from Baseline in RBS >= 1 point and/or RBS = 0 or 1. HEMI is defined as MES = 0 or 1 and Geboes Index score <3.1 Symptomatic remission is defined as RBS=0 and SFS= 0 or 1 †For FDA testing protocol, symptomatic remission was an “other secondary” endpoint, not multiplicity controlled * Endoscopic improvement/symptomatic remission were co-primary endpoints for the EMA protocol and were met by both doses in both trials ‡Hierarchical testing strategy was used starting with 50mg for the primary endpoint followed by the key secondary endpoints; the 25mg was subsequently tested for the primary endpoint followed by the key secondary endpoints.

Table: Treatment-emergent adverse events (TEAEs) throughout 8 weeks in ABTECT-1 and ABTECT-2 Phase 3 trials
Disclosures:
Bruce Sands: AbbVie; Consultant. Abivax; Consultant. Adiso Therapeutics; Consultant. AgomAb; Consultant. Alimentiv; Consultant. Amgen; Consultant. AnaptysBio; Consultant. Arena; Consultant. Artugen Therapeutics; Consultant. AstraZeneca; Consultant. Biolojic Design; Consultant. Biora Therapeutics; Consultant. Boehringer Ingelheim; Consultant. Bristol Meyer Squibb; Consultant. Celltrion; Consultant. Enthera; Consultant. Enveda Biosciences; Consultant. Equillium; Consultant. Evommune; Consultant. Ferring; Consultant. Fzata; Consultant. Galapagos; Consultant. Genentech-Roche; Consultant. Gilead; Consultant. Gossamer Bio; Consultant. GSK; Consultant. Imhotex; Consultant. Index Pharmaceuticals; Consultant. Innovation Pharmaceuticals; Consultant. Janssen; Consultant. Kaleido; Consultant. Kallyope; Consultant. Lilly; Consultant. Merck; Consultant. Microba; Consultant. Microbiotica; Consultant. Mitsubishi Tanabe; Consultant. Mobius Care; Consultant. Morphic Therapeutics; Consultant. MRM Health; Consultant. Nexus Therapeutics; Consultant. Nimbus Discovery; Consultant. Odyssey Therapeutics; Consultant. OSE Immunotherapeutics; Consultant. Palisade Bio; Consultant. Pfizer; Consultant. Protagonist Therapeutics; Consultant. Q32 Bio; Consultant. Rasayana Therapeutics; Consultant. Recludix Therapeutics; Consultant. Reistone Biopharma; Consultant. Sanofi; Consultant. Sorriso Therapeutics; Consultant. Spyre Therapeutics; Consultant. Takeda; Consultant. Target RWE; Consultant. Teva; Consultant. TLL Pharmaceutical; Consultant. Tr1X; Consultant. Trex Bio; Consultant. Union Therapeutics; Consultant. Ventyx Biosciences; Consultant.
Silvio Danese: AbbVie; Consultant. Allergan; Consultant. Biogen; Consultant. Boehringer Ingelheim; Consultant. Celgene; Consultant. Celltrion; Consultant. Ferring; Consultant. Hospira; Consultant. Johnson & Johnson; Consultant. Merck; Consultant. MSD; Consultant. Mundipharma; Consultant. Pfizer Inc; Consultant. Sandoz; Consultant. Takeda; Consultant. Tigenix; Consultant. UCB Pharma; Consultant. Vifor; Consultant.
Laurent Peyrin-Biroulet: AbbVie; Consultant. Abivax; Consultant. Adacyte; Consultant. Alimentiv; Consultant. Amgen; Consultant. Applied Molecular Transport; Consultant. Arena; Consultant. Banook; Consultant. Biogen; Consultant. BMS; Consultant. Celltrion; Consultant, Grant/Research Support. Connect Biopharm; Consultant. Cytoki Pharma; Consultant. Enthera; Consultant. Ferring; Consultant. Fresenius Kabi; Consultant, Grant/Research Support. Galapagos; Consultant. Genentech; Consultant. Gossamer Bio; Consultant. GSK; Consultant. IAC Image Analysis; Consultant. Index Pharmaceuticals; Consultant. Inotrem; Consultant. Janssen; Consultant. Lilly; Consultant. Medac; Consultant, Grant/Research Support. Mopac; Consultant. Morphic; Consultant. MSD; Consultant, Grant/Research Support. Nordic Pharma; Consultant. Novartis; Consultant. Oncodesign; Consultant. ONO Pharma; Consultant. OSE Immunotherapeutics; Consultant. Pandion Therapeuthics; Consultant. Par' Immune; Consultant. Pfizer; Consultant. Prometheus; Consultant. Protagonist; Consultant. Roche; Consultant. Samsung; Consultant. Sandoz; Consultant. Sanofi; Consultant. Satisfay; Consultant. Takeda; Consultant, Grant/Research Support. Telavant; Consultant. Theravance; Consultant. Thermo Fischer; Consultant. Tigenix; Consultant. Tillots; Consultant. Vectivbio; Consultant. Ventyx; Consultant. Viatris; Consultant. Ysopia; Consultant.
Marla Dubinsky: AbbVie; Consultant. Abivax; Consultant. Arena Pharmaceuticals; Consultant. AstraZeneca; Consultant. Boehringer Ingelheim; Consultant. Bristol Meyer Squibb; Consultant. F. Hoffmann-La Roche Ltd; Consultant. Genentech; Consultant. Gilead; Consultant. Janssen; Consultant. Lilly; Consultant. Merck; Consultant. Pfizer Inc; Consultant. Prometheus; Consultant. Takeda; Consultant.
Tadakazu Hisamatsu: AbbVie; Consultant, Grant/Research Support. Abivax; Consultant. Boston Scientific Corporation; Grant/Research Support. Bristol Meyer Squibb; Consultant. EA pharma; Consultant, Grant/Research Support. Gilead; Consultant. Janssen; Consultant. JIMRO Co. Ltd; Grant/Research Support. Kissei Pharmaceutical; Grant/Research Support, Lecture fee. Kyorin Pharmaceutical; Grant/Research Support. Lilly; Consultant. Mitsubishi Tanabe; Consultant, Grant/Research Support. Mochida Pharmaceutical; Grant/Research Support. Nippon Kayaku; Grant/Research Support. Pfizer Inc; Consultant, Grant/Research Support. Takeda; Grant/Research Support. Zeria Pharmaceutical Co. Ltd; Grant/Research Support.
Herbert Tilg: AbbVie; Consultant. Abivax; Consultant. Dr Falk Pharma; Consultant. Ferring; Consultant. Galapagos; Consultant. Microbiotica; Consultant. MSD; Consultant. Pfizer; Consultant. Takeda; Consultant.
Raja Atreya: AbbVie; Consultant. Abivax; Consultant. AstraZeneca; Consultant. Bristol Meyer Squibb; Consultant. Celltrion; Consultant. Galapagos; Consultant. Johnson & Johnson; Consultant. Lilly; Consultant. MSD; Consultant. Pfizer; Consultant. Takeda; Consultant.
Alessandro Armuzzi: AbbVie; Consultant. Abivax; Consultant. Alpha Sigma; Consultant. AstraZeneca; Consultant. Biogen; Consultant, Grant/Research Support. Boehringer Ingelheim; Consultant. Bristol Meyer Squibb; Consultant. Celltrion; Consultant. Enthera; Consultant. Ferring; Consultant. Galapagos; Consultant. Gilead; Consultant. Giuliani; Consultant. Janssen; Consultant. Lilly; Consultant. Lionhealth; Consultant. MSD; Consultant, Grant/Research Support. Nestlé; Consultant. Pfizer; Consultant, Grant/Research Support. Protagonist; Consultant. Roche; Consultant. Samsung Bioepis; Consultant. Sandoz; Consultant. Sanofi; Consultant. Takeda; Consultant, Grant/Research Support. Teva; Consultant. Tillots; Consultant.
Xavier Treton: AbbVie; Consultant. Abivax; Consultant. Alpha Sigma; Consultant. Biogen; Consultant. Celltrion; Consultant. Dr Falk Pharma; Consultant. Fresenius Kabi; Consultant. Johnson & Johnson; Consultant. Lilly; Consultant. MSD; Consultant. Pfizer; Consultant. Takeda; Consultant. Thabor Therapeutics; Consultant. Tillots; Consultant.
Filip Baert: AbbVie; Consultant, Grant/Research Support. Amgen; Consultant, Grant/Research Support. Arena Pharmaceuticals; Consultant. Celgene; Consultant. Celltrion; Consultant. Eurogenerics; Grant/Research Support. Ferring; Consultant. Fresenius Kabi; Consultant. Johnson & Johnson; Consultant, Grant/Research Support. MSD; Consultant. Pfizer Inc; Consultant. Sandoz; Consultant. Takeda; Grant/Research Support.
Ursula Seidler: AbbVie; Consultant, Grant/Research Support. Abivax; Consultant, Grant/Research Support. Amgen; Consultant. Boehringer Ingelheim; Grant/Research Support. Bristol Meyer Squibb; Grant/Research Support. Celgene; Grant/Research Support. Galapagos; Consultant, Grant/Research Support. Gilead; Grant/Research Support. Janssen; Consultant, Grant/Research Support. Lilly; Consultant, Grant/Research Support. Pfizer; Grant/Research Support. Roche; Grant/Research Support. Takeda; Grant/Research Support.
Fabio Cataldi: Abivax; Employee.
Doug Jacobstein: Abivax; Employee.
Christopher J. Rabbat: Abivax; Employee.
Kejia Shan: Abivax; Employee.
George Aaron Duvall indicated no relevant financial relationships.
Britta Siegmund: AbbVie; Consultant. Abivax; Consultant. Boehringer Ingelheim; Consultant. Bristol Meyer Squibb; Consultant. Dr Falk Pharma; Consultant. Endpoint Health; Consultant. Falk; Consultant. Galapagos; Consultant. Gilead; Consultant. Janssen; Consultant. Landos; Consultant. Lilly; Consultant. Materia Prima; Consultant. Pfizer; Consultant, Grant/Research Support. Predictimmune; Consultant. Takeda; Consultant.
Parambir Dulai: AbbVie; Consultant. Abivax; Consultant. Adiso; Consultant. Alimentiv; Consultant. Bristol Meyer Squibb; Consultant. Celltrion; Consultant. Genentech; Consultant. Geneoscopy; Consultant. Janssen; Consultant. Pfizer; Consultant. Takeda; Consultant.
David Rubin: AbbVie; Consultant. Abivax; Consultant. Altrubio; Consultant. Athos Therapeutics; Consultant. Bristol Meyer Squibb; Consultant. Celltrion; Consultant. Connect Biopharma; Consultant. Genentech; Consultant. Iterative Health; Consultant. Janssen; Consultant. Johnson & Johnson; Consultant. Lilly; Consultant. Merck; Consultant. Odyssey Therapeutics; Consultant. Pfizer; Consultant. Sanofi; Consultant. Spyre; Consultant. Takeda; Consultant, Grant/Research Support. Vedanta Biosciences; Consultant. Ventyx Biosciences; Consultant.
Séverine Vermeire: AbbVie; Consultant, Grant/Research Support. Abivax; Consultant. AbolerIS Pharma; Consultant. AgomAb; Consultant. Alimentiv; Consultant. Arena; Consultant. AstraZeneca; Consultant. Avaxia; Consultant. BMS; Consultant. Boehringer Ingelheim; Consultant. Celgene; Consultant. CVasThera; Consultant. Cytoki Pharma; Consultant. Dr Falk Pharma; Consultant. Ferring; Consultant. Galapagos; Consultant, Grant/Research Support. Genentech-Roche; Consultant. Gilead; Consultant. GSK; Consultant. Hospira; Consultant. Imidomics; Consultant. Janssen; Consultant. Johnson & Johnson; Consultant, Grant/Research Support. Lilly; Consultant. Materia Prima; Consultant. MiroBio; Consultant. Morphic; Consultant. MRM Health; Consultant. MSD; Consultant. Mundipharma; Consultant. Pfizer; Consultant, Grant/Research Support. Prodigest; Consultant. Progenity; Consultant. Prometheus; Consultant. Robarts Clinical Trials; Consultant. Second Genome; Consultant. Shire; Consultant. Surrozen; Consultant. Takeda; Consultant, Grant/Research Support. Theravance; Consultant. Tillots; Consultant. Zealand Pharma; Consultant.

