Monday Poster Session
Category: IBD
P3338 - Treatment Discontinuation in Inflammatory Bowel Disease (IBD) Patients Receiving Biologic Therapies That Require Intravenous (IV) Induction and Subcutaneous (SC) Maintenance Using Two Large US Claims Databases
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Jennifer Seminerio-Diehl, MD (she/her/hers)
Director Inflammatory Bowel Disease
Department of Gastroenterology and Hepatology, AdventHealth Orlando
Orlando, FL
Presenting Author(s)
Jennifer Seminerio, MD1, Yichen Zhang, PhD, MPH2, Stephen Uong, MPH2, Shashi Adsul, MD, MBA, MBBS3, Gustavo Scapini, MD, PhD, MS, MBA3, Christopher Busse, MS3, Sheetal P. Dharia, PharmD, PhD3, Alice Xiaoxiao Lu, MD, PhD, MPH2, Ryan Ungaro, MD, MS4
1Department of Gastroenterology and Hepatology, AdventHealth Orlando, Orlando, FL; 2Johnson & Johnson, Raritan, NJ; 3Johnson & Johnson, Horsham, PA; 4Dr. Henry D. Janowitz Division of Gastroenterology, Icahn School of Medicine at Mount Sinai, New York, NY
Introduction: Adherence to biologic therapies in patients with ulcerative colitis (UC) or Crohn’s disease (CD) can be challenging, particularly for those undergoing an intravenous (IV) infusion induction followed by subcutaneous (SC) maintenance doses. This difficulty is partly due to the need for multiple prescriptions and prior authorizations from health insurance providers for IV and SC doses. This study assessed the discontinuation rates of biologic therapies that involve IV induction and SC maintenance dosing in patients with UC or CD in the US prior to starting maintenance therapy.
Methods: A retrospective cohort study used claims from de-identified HealthVerity (HV) TaXonomy(X) (1/1/2021-7/31/2024), including closed medical and pharmacy claims from multiple data providers, and Optum Clinformatics® Data Mart Database (1/1/2021-6/30/2024), including claims solely from UnitedHealth. Treatment discontinuation was defined as a gap ≥ 2.5 times the dosing interval between two consecutive doses in the FDA label.
Results: 19,777 patients were identified in HV: 2,869 treated with risankizumab (RZB), and 16,908 with ustekinumab (UST). 3,261 were identified in Optum: 635 with RZB, 2,626 with UST. No mirikizumab-treated patients were identified in either database. Baseline characteristics were similar across two databases (Table 1). Among patients with FDA-labeled numbers of IV infusions, 23.5% and 24.2% of the RZB patients in HV and Optum respectively, discontinued during the transition from induction to maintenance therapy. Among the UST patients, 16.1% in HV and 19.9% in Optum discontinued during the transition (Figure 1). By indication, the discontinuation rate was 17.7% among CD patients and 15.5% for UC patients in HV (21.2% and 18.9% in Optum respectively).
Discussion: In patients with UC or CD treated with UST or RZB, around one-fifth of patients discontinued during the transition from induction to maintenance, a finding consistent in both databases. These findings emphasize that the route of administration may pose a significant challenge in maintaining patients on injectable biologics. Considerations regarding adherence should be given when deciding the route of administration of a drug, with a fully SC drug potentially offering advantages in treatment continuity.
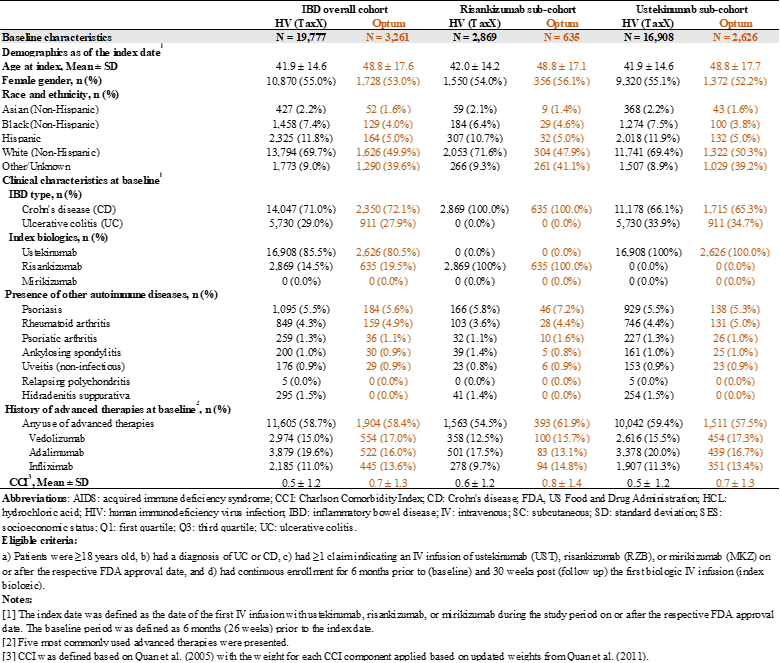
Figure: Table 1. Baseline Characteristics in Overall and By Index Biologic in Each Database
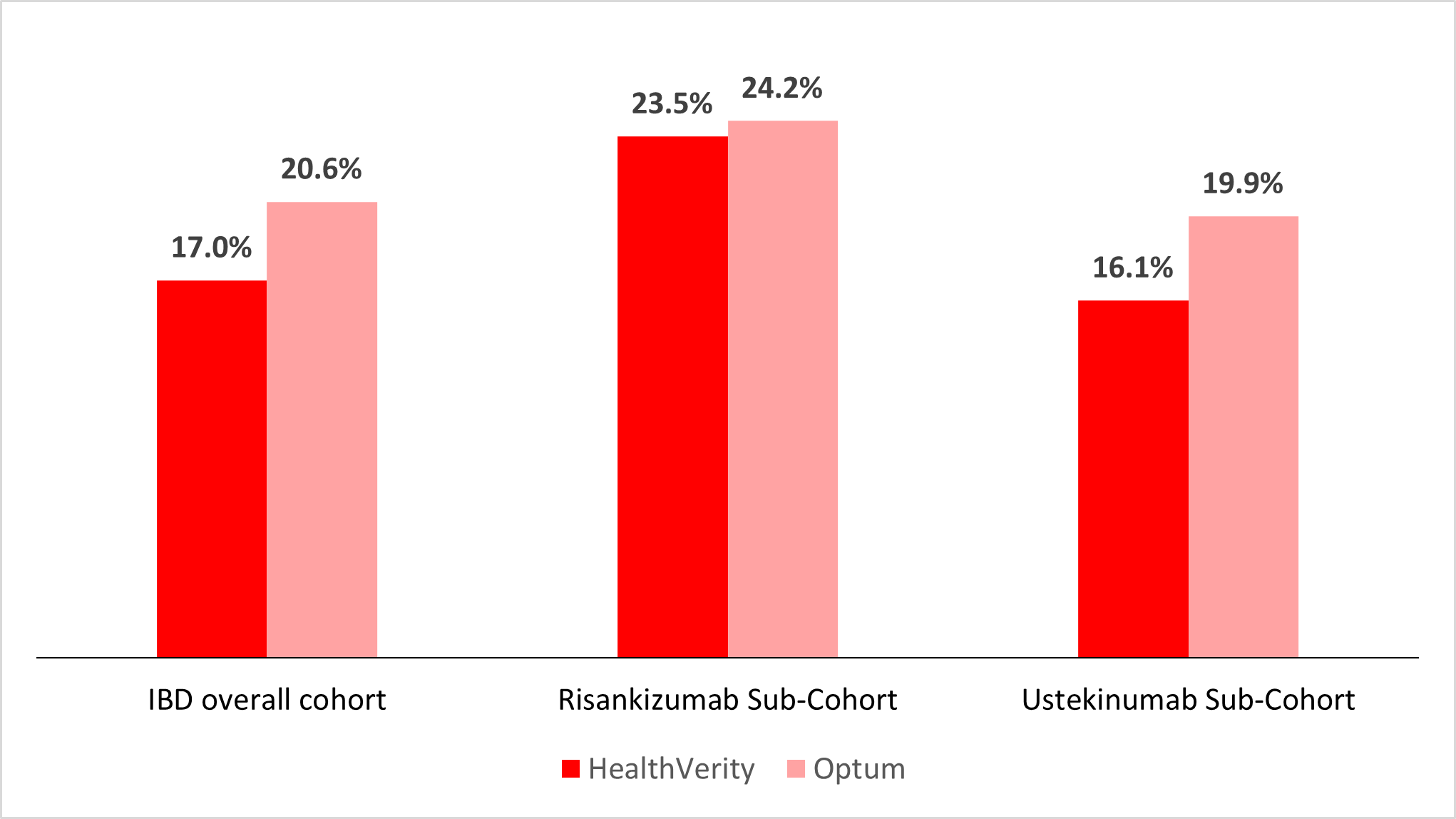
Figure: Figure 1. Treatment Discontinuation in Patients Transitioning from FDA Labeled Intravenous (IV) Induction Doses to Subcutaneous (SC) Maintenance
Disclosures:
Jennifer Seminerio: Abbvie – Consultant. Johnson & Johnson – Consultant. Lilly – Consultant. Pfizer – Consultant. Sanofi – Consultant. Takeda – Consultant.
Yichen Zhang: BioMarin Pharmaceutical Inc. – Employee. Johnson & Johnson – Employee.
Stephen Uong: Johnson & Johnson – Employee, Independent Contractor, Technically contracted with a third-party company with J&J, but functionally working as a J&J employee..
Shashi Adsul indicated no relevant financial relationships.
Gustavo Scapini: Johnson & Johnson – Employee.
Christopher Busse: Johnson & Johnson – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Sheetal P. Dharia: Johnson & Johnson – Employee, Stock-publicly held company(excluding mutual/index funds).
Alice Xiaoxiao Lu: Johnson & Johnson – Employee, Stock-publicly held company(excluding mutual/index funds).
Ryan Ungaro: AbbVie – Advisory Committee/Board Member, Consultant, Grant/Research Support. Boehringer Ingelheim – Grant/Research Support. Bristol Myers Squibb – Advisory Committee/Board Member, Consultant. Janssen – Advisory Committee/Board Member, Consultant. Lilly – Grant/Research Support. Pfizer – Advisory Committee/Board Member, Consultant, Grant/Research Support. Takeda – Advisory Committee/Board Member, Consultant.
Jennifer Seminerio, MD1, Yichen Zhang, PhD, MPH2, Stephen Uong, MPH2, Shashi Adsul, MD, MBA, MBBS3, Gustavo Scapini, MD, PhD, MS, MBA3, Christopher Busse, MS3, Sheetal P. Dharia, PharmD, PhD3, Alice Xiaoxiao Lu, MD, PhD, MPH2, Ryan Ungaro, MD, MS4. P3338 - Treatment Discontinuation in Inflammatory Bowel Disease (IBD) Patients Receiving Biologic Therapies That Require Intravenous (IV) Induction and Subcutaneous (SC) Maintenance Using Two Large US Claims Databases, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Department of Gastroenterology and Hepatology, AdventHealth Orlando, Orlando, FL; 2Johnson & Johnson, Raritan, NJ; 3Johnson & Johnson, Horsham, PA; 4Dr. Henry D. Janowitz Division of Gastroenterology, Icahn School of Medicine at Mount Sinai, New York, NY
Introduction: Adherence to biologic therapies in patients with ulcerative colitis (UC) or Crohn’s disease (CD) can be challenging, particularly for those undergoing an intravenous (IV) infusion induction followed by subcutaneous (SC) maintenance doses. This difficulty is partly due to the need for multiple prescriptions and prior authorizations from health insurance providers for IV and SC doses. This study assessed the discontinuation rates of biologic therapies that involve IV induction and SC maintenance dosing in patients with UC or CD in the US prior to starting maintenance therapy.
Methods: A retrospective cohort study used claims from de-identified HealthVerity (HV) TaXonomy(X) (1/1/2021-7/31/2024), including closed medical and pharmacy claims from multiple data providers, and Optum Clinformatics® Data Mart Database (1/1/2021-6/30/2024), including claims solely from UnitedHealth. Treatment discontinuation was defined as a gap ≥ 2.5 times the dosing interval between two consecutive doses in the FDA label.
Results: 19,777 patients were identified in HV: 2,869 treated with risankizumab (RZB), and 16,908 with ustekinumab (UST). 3,261 were identified in Optum: 635 with RZB, 2,626 with UST. No mirikizumab-treated patients were identified in either database. Baseline characteristics were similar across two databases (Table 1). Among patients with FDA-labeled numbers of IV infusions, 23.5% and 24.2% of the RZB patients in HV and Optum respectively, discontinued during the transition from induction to maintenance therapy. Among the UST patients, 16.1% in HV and 19.9% in Optum discontinued during the transition (Figure 1). By indication, the discontinuation rate was 17.7% among CD patients and 15.5% for UC patients in HV (21.2% and 18.9% in Optum respectively).
Discussion: In patients with UC or CD treated with UST or RZB, around one-fifth of patients discontinued during the transition from induction to maintenance, a finding consistent in both databases. These findings emphasize that the route of administration may pose a significant challenge in maintaining patients on injectable biologics. Considerations regarding adherence should be given when deciding the route of administration of a drug, with a fully SC drug potentially offering advantages in treatment continuity.

Figure: Table 1. Baseline Characteristics in Overall and By Index Biologic in Each Database

Figure: Figure 1. Treatment Discontinuation in Patients Transitioning from FDA Labeled Intravenous (IV) Induction Doses to Subcutaneous (SC) Maintenance
Disclosures:
Jennifer Seminerio: Abbvie – Consultant. Johnson & Johnson – Consultant. Lilly – Consultant. Pfizer – Consultant. Sanofi – Consultant. Takeda – Consultant.
Yichen Zhang: BioMarin Pharmaceutical Inc. – Employee. Johnson & Johnson – Employee.
Stephen Uong: Johnson & Johnson – Employee, Independent Contractor, Technically contracted with a third-party company with J&J, but functionally working as a J&J employee..
Shashi Adsul indicated no relevant financial relationships.
Gustavo Scapini: Johnson & Johnson – Employee.
Christopher Busse: Johnson & Johnson – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Sheetal P. Dharia: Johnson & Johnson – Employee, Stock-publicly held company(excluding mutual/index funds).
Alice Xiaoxiao Lu: Johnson & Johnson – Employee, Stock-publicly held company(excluding mutual/index funds).
Ryan Ungaro: AbbVie – Advisory Committee/Board Member, Consultant, Grant/Research Support. Boehringer Ingelheim – Grant/Research Support. Bristol Myers Squibb – Advisory Committee/Board Member, Consultant. Janssen – Advisory Committee/Board Member, Consultant. Lilly – Grant/Research Support. Pfizer – Advisory Committee/Board Member, Consultant, Grant/Research Support. Takeda – Advisory Committee/Board Member, Consultant.
Jennifer Seminerio, MD1, Yichen Zhang, PhD, MPH2, Stephen Uong, MPH2, Shashi Adsul, MD, MBA, MBBS3, Gustavo Scapini, MD, PhD, MS, MBA3, Christopher Busse, MS3, Sheetal P. Dharia, PharmD, PhD3, Alice Xiaoxiao Lu, MD, PhD, MPH2, Ryan Ungaro, MD, MS4. P3338 - Treatment Discontinuation in Inflammatory Bowel Disease (IBD) Patients Receiving Biologic Therapies That Require Intravenous (IV) Induction and Subcutaneous (SC) Maintenance Using Two Large US Claims Databases, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
